Hi, I have a test for concentration of NH4Cl, but need to know the concentration NH3. I've been digging for information/calculations about it for at least 3 hours and this is what I come up with:
Test results: NH4Cl - 250ppm
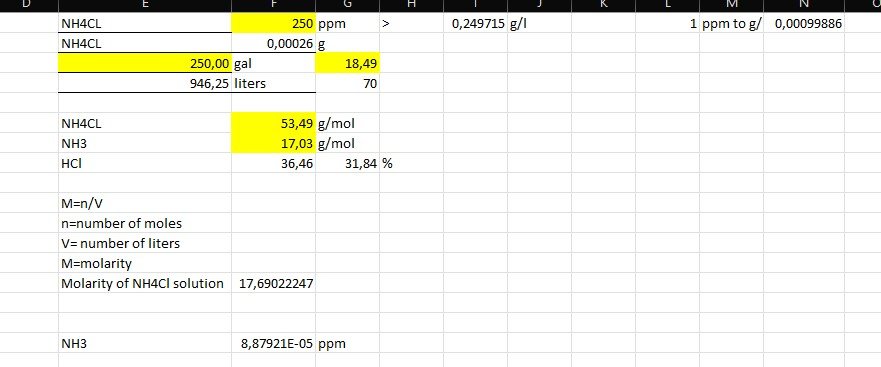
I did excel calculations, seen below, on how to get from NH4Cl grams to NH3 ppm, but the tricky part is that i only know ppm and also i didn't account much for pH.
Is it even possible to calculate without any more additional testing or other info?
This similar topic was discussed also here:

 www.reef2reef.com
www.reef2reef.com
Thanks,
Adam
Test results: NH4Cl - 250ppm
I did excel calculations, seen below, on how to get from NH4Cl grams to NH3 ppm, but the tricky part is that i only know ppm and also i didn't account much for pH.
Is it even possible to calculate without any more additional testing or other info?
This similar topic was discussed also here:

Ammonium Chloride Addition Calculation
Can someone double check my calculation on this? If I add 1.5 grams of Ammonium Chloride to 48 gallons of saltwater water, what should the ammonia concentration in ppm be once it is mixed? This is what I calculated (neglecting any NH3 loss to atmosphere). MW of NH4Cl = 53.49 g/mol in solution...
 www.reef2reef.com
www.reef2reef.com
Thanks,
Adam





















