The man, the myth, the legend!
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Aquarium Chemistry Question? Ask the Doctor!
- Thread starter revhtree
- Start date
- Tagged users None
- Status
- Not open for further replies.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
Dear Dr. Randy,
When we talk about alkalinity in a reef tank, is it Alkalinity (M), Alkalinity (P) or Alkalinity (Total)?
Best,
Rabih
I started a new thread on this topic here:
https://www.reef2reef.com/threads/alkalinity-in-a-reef-tank.243909/
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
revhtree
Owner Administrator
View Badges

Staff member
Super Moderator
Reef Squad
Excellence Award
RGB
Photo of the Month
Article Contributor
R2R TV Featured
Hospitality Award
Article Administrator
Black Friday Sponsor
Industry Professional
My Tank Thread
My Aquarium Showcase
- Joined
- May 8, 2006
- Messages
- 49,257
- Reaction score
- 98,217
Ha, thanks, I hope.
You are....lol
- Joined
- Dec 20, 2015
- Messages
- 240
- Reaction score
- 158
Randy,
Not a question but a compliment. I've read a ton of your postings and articles over the last few months. They're been incredibly helpful and our two reefs are doing better than ever. You and @Humblefish are awesome!
Thank You!
Not a question but a compliment. I've read a ton of your postings and articles over the last few months. They're been incredibly helpful and our two reefs are doing better than ever. You and @Humblefish are awesome!
Thank You!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
Thanks!
Happy Reefing.
Happy Reefing.
I have been reading a bit on nitrates lately. most people seem to want their tanks to hover around undetectable levels. however I ran across an article that suggested that the zero level was not ideal. That in heavily populated sps tanks the corals could literately deplete the tank of nitrates, and would benefit from higher levels. i read another article that said that nitrate levels were not a problem, as much as phosphate levels because the phosphates inhibit the deposition of calcium in the coral skeleton. any thoughts? trying to remember if i saved those articles.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
Very low levels may not always be desirable. I recommend 0.25 - 2 ppm if you are not heavily feeding organic foods suitable for corals.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
I have a planet aquarium 65G tank with 35g sump. I just buying dosing and dont know how much ml/day or week i need to dose. As far as alkalinity and mg. how much ml i need to set it up?
The answer depends entirely on the demand in the tank. You need to get an alkalinity kit, measure it, and see what you need (if any).
Thank you for the article on PH and for everything you do. I always learns so much from your teachings. I am battling a steady rise in PH. About four months ago my PH swung from 8-8.25. Steadily its been going up to 8.3-8.5. I know its still in range and I haven't seen any bad effects from it but if it continues It could spell trouble, so I thought I would vinegar dose to try and get it back down. Might do 2 things for me as my nitrates are at 5 ppm as well. I started this evening while my lights are on putting in about 50 ml in 120 gallons over the course of an hour. My PH was 8.48 when I started and dropped to 8.38 in about a 2 hour time frame. Is that too fast of a drop? Also my alk hovers around 7 dkh (which is a little lower than I want) cal at 420 and mag at 1320, all tested today before I dosed the vinegar. By the way I test the PH using my apex probe which I calibrate about every 3 months and confirmed it today with a test kit as well before I dosed. I dose BRS 2 part at a rate of 30 ml per day. when I go higher than that my alk rarely changes but my cal gets out of hand high (500+).
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
Thank you for the article on PH and for everything you do. I always learns so much from your teachings. I am battling a steady rise in PH. About four months ago my PH swung from 8-8.25. Steadily its been going up to 8.3-8.5. I know its still in range and I haven't seen any bad effects from it but if it continues It could spell trouble, so I thought I would vinegar dose to try and get it back down. Might do 2 things for me as my nitrates are at 5 ppm as well. I started this evening while my lights are on putting in about 50 ml in 120 gallons over the course of an hour. My PH was 8.48 when I started and dropped to 8.38 in about a 2 hour time frame. Is that too fast of a drop? Also my alk hovers around 7 dkh (which is a little lower than I want) cal at 420 and mag at 1320, all tested today before I dosed the vinegar. By the way I test the PH using my apex probe which I calibrate about every 3 months and confirmed it today with a test kit as well before I dosed. I dose BRS 2 part at a rate of 30 ml per day. when I go higher than that my alk rarely changes but my cal gets out of hand high (500+).
In the absence of high pH additives (notably limewater/kalkwasser), pH does not get high enough to ever warrant action, and if it appears to, it is probably testing error.
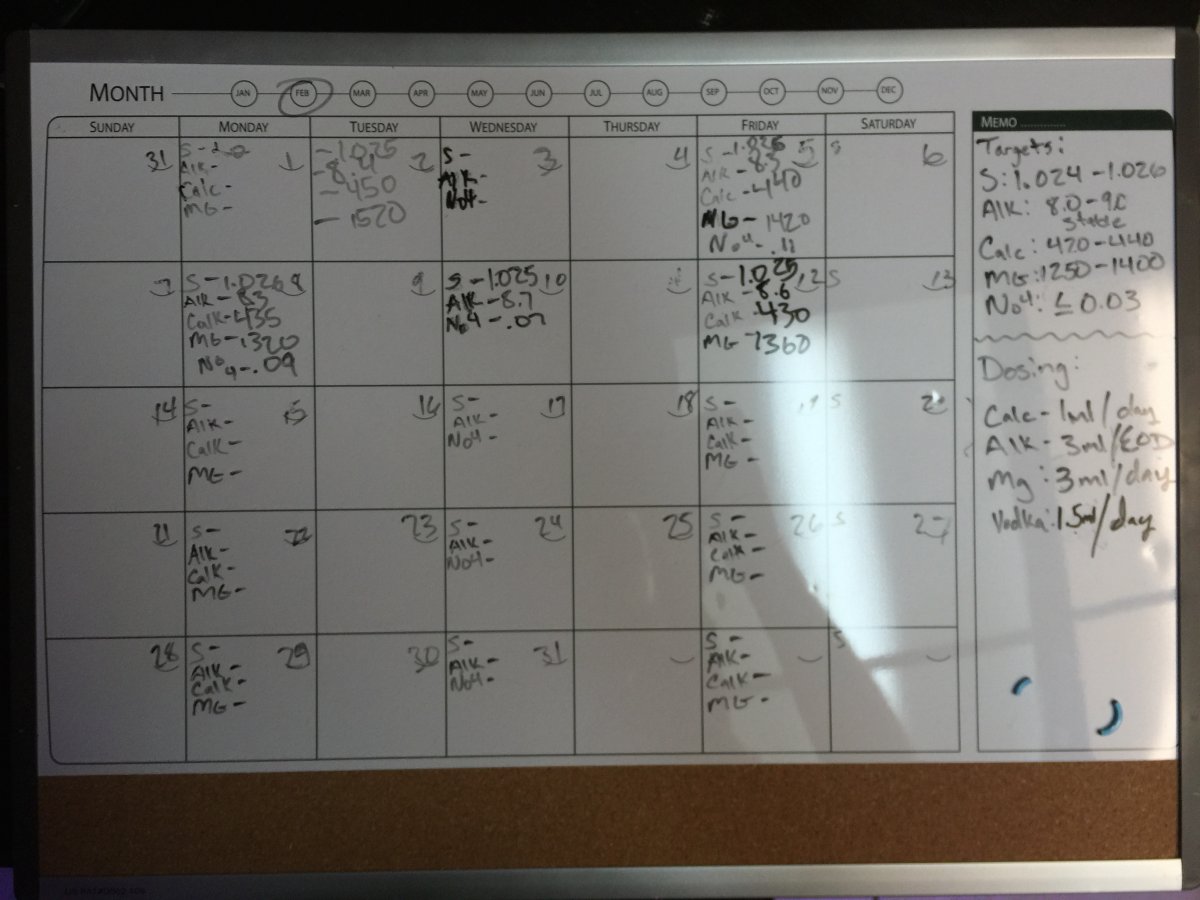
Any input on my parameters? I know my Mag spiked a little high recently but has slowly been dropping, and I believe it's stable at 1320-1360
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
the numbers for thee 12th look fine. 
My sand is starting to get hard (calcify?) in some parts of my tank. What could be the cause of this?
120g with 35g sump. Tank is almost 4 years old. Switched out sand a little over a year ago. Dose BRS Alk and Calcium.
Thanks!
120g with 35g sump. Tank is almost 4 years old. Switched out sand a little over a year ago. Dose BRS Alk and Calcium.
Thanks!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
My sand is starting to get hard (calcify?) in some parts of my tank. What could be the cause of this?
120g with 35g sump. Tank is almost 4 years old. Switched out sand a little over a year ago. Dose BRS Alk and Calcium.
Thanks!
Here's a thread where we discuss sand hardening:
https://www.reef2reef.com/threads/sand-bed-turning-rock-hard.183612/#post-2113274
Hey @Randy Holmes-Farley can you start a conversation with me? I can't start one with you 
@Randy Holmes-Farley
I am building an iOS application that allows users to track parameter measurements. I currently have 48 parameters that can be logged. I am having some difficulty while trying to research what units of measurement the parameters are measured in. I know that alkalinity can be measured in meq/L, dKH, and ppm. I want users of the application to not worry about conversions, and instead, just enter the amount and select the unit of measurement. The values can then be automatically converted and displayed to them in the log.
Is there a good place that you can point me to that will allow me to find the proper units of measurement? I realize that most of the parameters are in ppm, but I want to ensure that I am able to retrieve all possible and common units of measurement for the parameters. There are some, such as temperature, salinity, and others that I am confident about, but I am not about many of the others.
My current list of parameters is as follows:
Alkalinity, Aluminum, Ammonia, Antimony, Arsenic, Barium, Beryllium, Boron, Bromine, Cadmium, Calcium, Chromium, Chloramine, Chlorine (Free), Chlorine (Total), Cobalt, Copper, Iodine, Iron, Lead, Lithium, Magnesium, Manganese, Mercury, Molybdenum, Nickel, Nitrate, Nitrite, Oxidation-Reduction Potential, Oxygen, pH, Phosphate, Phosphorus, Photosynthetically Active Radiation, Potassium, Salinity, Selenium, Silica, Silicon, Sodium, Strontium, Sulfur, Temperature, Tin, Titanium, Total Dissolved Solids, Vanadium, Zinc
Attached are a couple of photos that maybe better explain what I am trying to achieve: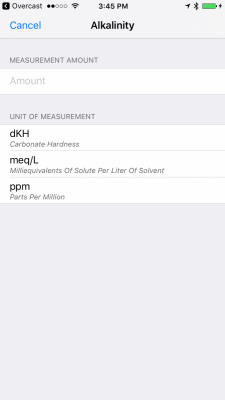
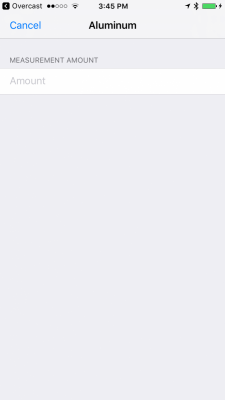
I am building an iOS application that allows users to track parameter measurements. I currently have 48 parameters that can be logged. I am having some difficulty while trying to research what units of measurement the parameters are measured in. I know that alkalinity can be measured in meq/L, dKH, and ppm. I want users of the application to not worry about conversions, and instead, just enter the amount and select the unit of measurement. The values can then be automatically converted and displayed to them in the log.
Is there a good place that you can point me to that will allow me to find the proper units of measurement? I realize that most of the parameters are in ppm, but I want to ensure that I am able to retrieve all possible and common units of measurement for the parameters. There are some, such as temperature, salinity, and others that I am confident about, but I am not about many of the others.
My current list of parameters is as follows:
Alkalinity, Aluminum, Ammonia, Antimony, Arsenic, Barium, Beryllium, Boron, Bromine, Cadmium, Calcium, Chromium, Chloramine, Chlorine (Free), Chlorine (Total), Cobalt, Copper, Iodine, Iron, Lead, Lithium, Magnesium, Manganese, Mercury, Molybdenum, Nickel, Nitrate, Nitrite, Oxidation-Reduction Potential, Oxygen, pH, Phosphate, Phosphorus, Photosynthetically Active Radiation, Potassium, Salinity, Selenium, Silica, Silicon, Sodium, Strontium, Sulfur, Temperature, Tin, Titanium, Total Dissolved Solids, Vanadium, Zinc
Attached are a couple of photos that maybe better explain what I am trying to achieve:


Last edited:
I have a question. Why does my ph rise to 8.47 during lights on and then It drops to 7.98 at night until my lights go back on. I'm using xr15pro with ramp up and ramp down. My peak is from 2pm to 5pm.
- Status
- Not open for further replies.
Similar threads
- Replies
- 4
- Views
- 128
- Replies
- 9
- Views
- 549
- Replies
- 9
- Views
- 520
- Replies
- 25
- Views
- 1,044
TOP 10 Trending Threads
- Replies
- 48
- Views
- 512
- Question
- Replies
- 65
- Views
- 683
- Replies
- 36
- Views
- 378
- Replies
- 31
- Views
- 398
-
- Poll
- Replies
- 31
- Views
- 446
- Replies
- 106
- Views
- 1,744
- Replies
- 29
- Views
- 473
- Replies
- 26
- Views
- 369



















