- Joined
- Sep 21, 2018
- Messages
- 6,684
- Reaction score
- 7,175
Aragonite Sand And Phosphate Adsorption. Alkalinity And pH Observations
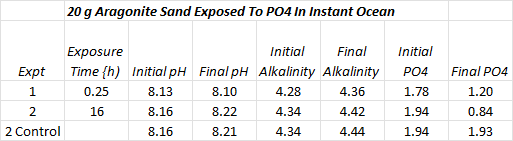
I ran two experiments using the same sand. The experiments consisted of exposing the sand to phosphate in Instant Ocean followed by a series of Instant Ocean “washes” until no more phosphate was detected and then exposing the sand again to phosphate and then washing out the phosphate. The first exposure was 15 minutes and last was for 16 hours. Results are summarized in the table below just after each adsorption phase of the experiment. I added a control in the second experiment to determine whether pH and alkalinity readings were related to phosphate adsorption or just drifting for other reasons.
The pH changes might be random, up or down, but alkalinity is consistently increasing, though within the margin of error and more tellingly, increasing with the same magnitude in the control. Repeating this experiment with fresh sand might be interesting because first time adsorptions tend to be somewhat larger then subsequent adsorptions.
I ran two experiments using the same sand. The experiments consisted of exposing the sand to phosphate in Instant Ocean followed by a series of Instant Ocean “washes” until no more phosphate was detected and then exposing the sand again to phosphate and then washing out the phosphate. The first exposure was 15 minutes and last was for 16 hours. Results are summarized in the table below just after each adsorption phase of the experiment. I added a control in the second experiment to determine whether pH and alkalinity readings were related to phosphate adsorption or just drifting for other reasons.
The pH changes might be random, up or down, but alkalinity is consistently increasing, though within the margin of error and more tellingly, increasing with the same magnitude in the control. Repeating this experiment with fresh sand might be interesting because first time adsorptions tend to be somewhat larger then subsequent adsorptions.


















