You have a wine refractometer. Salt mixes I have used are usually 1/2 cup per gallon, then a little tweaking.
I make wine but use a hydrometer.
Probably happens !
“So let it be written, so let it be done”
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
You have a wine refractometer. Salt mixes I have used are usually 1/2 cup per gallon, then a little tweaking.
You have a wine refractometer. Salt mixes I have used are usually 1/2 cup per gallon, then a little tweaking.
I make wine but use a hydrometer.
Probably happens !
“So let it be written, so let it be done”
Lol. Randy has got a DIY refractometer calibration fluid using RODI and table salt, if you are interested.Mistakes were made new refractometer measures 1.041.. this is one for the books

Randy, we all know your reputation and who you are, but the way you come across on the forums leaves something to be desired. You've rubbed me the wrong way more than once, and I've seen you called out on it in multiple settings. You should probably take stock of those things and re-evaluate how you approach people.
Are you really that set in your ways that you won't accept a little criticism from an outside observer on *how* you interact with people? Your information is fine, but this shouldn't be the way you communicate with someone for the first time:Since both you and the OP do not care for my approach or the info I am providing, I will attend to other folks issues.
That's simply not correct.
I think you should stop randomly getting info from around the internet.
What is the evidence that you have problematic levels of ammonia? Smell only?

Since both you and the OP do not care for my approach or the info I am providing, I will attend to other folks issues.
You are of course welcome to your opinions about my responses. I have heard all sorts of criticisms and have made and admitted all sorts of mistakes worthy of criticism in the past 60 years. Other than being harsher than you prefer, which I am well aware of, I do not see anything worthy of my apologizing for, and I'm going to move on. I am not running for election or looking for likes. My main focus for decades has been, and will be, to correct incorrect chemical information that spreads like wildfire through the reefing community. Even if you do not appreciate the info because it wasn't delivered in a way you want to receive it, my role is completed as I believe I prevented others from reading and coming away with a mistaken impression of certain chemical facts.
That all said, this is not the place to debate my personality attributes, and I won't be responding further here. If it is important to you, feel free to start a new thread on this topic in my forum.

maybe you can log a comparison between Brix% refractometers salinity vs a dedicated saltwater refractometer. I didn’t see it on your how to use a refractometer, not that it should be on there…


You said nothing in your original response as to why it is incorrect, just that it was.
Not sure exactly what you mean by a comparison, but how to use one to get salinity is here:

Refractometers and Salinity Measurement - REEFEDITION
Title photo by R2R member Rskillz By Randy Holmes-Farley Salinity is one of the most important parameters measured in reef aquaria. It controls not only the salt balance between an organism and its surrounding environment, but also the levels of a host of ions Read more here...www.reefedition.com
Brix Refractometers
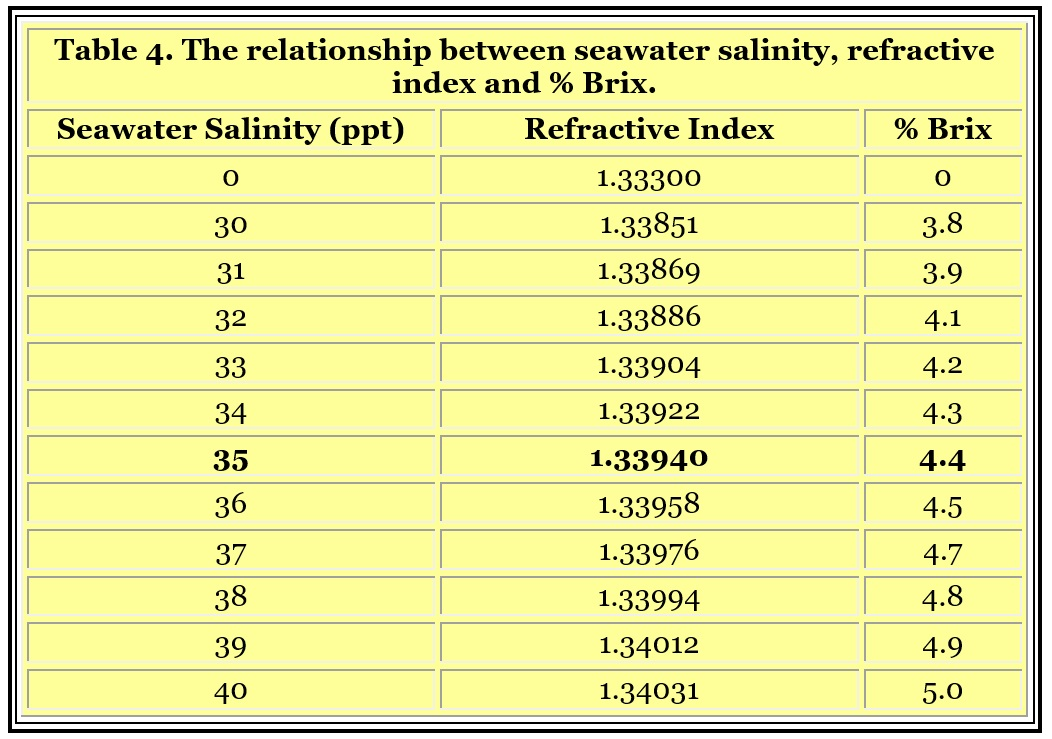
A commonly manufactured type of refractometer is called a Brix refractometer. Its scale usually reads in Brix, or % Brix (percent Brix). These refractometers are used in many industries to measure the concentration of sugar in water such as in the soft drink industry. They can be used to measure seawater’s salinity, but are not always precise enough around the range of seawater’s refractive index to be useful. A resolution of 0.2% Brix is common, and that is borderline acceptable for the reasons detailed below.
Table 4 shows the relationship between seawater salinity, refractive index and % Brix. If a refractometer has a resolution (not accuracy, but resolution, which is the finest amount it can distinguish) of 0.2 % Brix, then that translates to about +/- 1 ppt. So the best resolution would translate to 35 ppt seawater reading 34-36 ppt, which may be adequate for reef aquarists. A Brix refractometer that reads 0 to 10 % Brix with a resolution of 0.1% Brix might be a fine choice for determining seawater salinity in a reef aquarium, (although they are not inexpensive). Some Brix refractometers have a resolution of 0.5 % Brix or even 1% Brix, and they would not be suitable choices.

and of why the reagents in the API ammonia test bind to high salinity, whereas another wouldn’t.. other than knowing the intricacies of their chemicals… as in if the salinity is too high, will test results be tainted.

In other words, to interpret the measurement using the specific Brix I have, in the first image I attached, to get 35ppt (1.025) I would looking for 4.4 on the left, but 1.017~ on the right side of the scale. I did see this chart in your article/post but it didn’t make sense as to why there is what appears to be salinity values on the right side.. The 1.33 number threw off my thought process, assuming 1.033 in another way.
Getting the salinity within the range expected by the kit is important.
Both Nessler kits and Salicylate kits are interfered with by both calcium and magnesium, and the kits remove them. If salinity is high, those will be higher than the kits expect and the kit may not have the capacity to remove them. Thank you!
"Elevated calcium and magnesium (hardness) concentration and salinity can interfere with the determination of TAN concentration"
"Nessler's reagent raises the sample pH causing precipitation of calcium and magnesium (hardness cations) as hydroxides"
Was the CUC shipped? Trochus dont ship well and others may take a day or so to start moving.
I know this thread is quite old, but I just want to say that I'm having the same problem with the Salifert NH3 test kit while cycling. I looked at several reviews on Amazon and posts here on R2R. Apparently, many people also had the same issue. I used my Sera test kit and got a reading of 0.5 mg/l. The Salifert tests made me think that the cycle has finished since I couldn't detect any Nh3/Nh4 or NO2 in my water. I used Sera for my No3, and I was so confused when my NO3 was also 0. Now I understand that the Salifert NH3 tests are not to be trusted when cycling a new tank.Bought a second API test to see if the first was faulty. Same result. Salifert looks little to none, unless I’m off.

I know this thread is quite old, but I just want to say that I'm having the same problem with the Salifert NH3 test kit while cycling. I looked at several reviews on Amazon and posts here on R2R. Apparently, many people also had the same issue. I used my Sera test kit and got a reading of 0.5 mg/l. The Salifert tests made me think that the cycle has finished since I couldn't detect any Nh3/Nh4 or NO2 in my water. I used Sera for my No3, and I was so confused when my NO3 was also 0. Now I understand that the Salifert NH3 tests are not to be trusted when cycling a new tank.
Might as well use the Seachem Alert Badge for free ammonia.
I'm not sure which one is right or the scientific base behind their kits, but I checked the water separately with both kits using fresh saltwater and an ammoniac source. The Sera one gave me a reading, the Salifert one remained 0. I'm just saying that if one wants to cycle the tank, Salifert isn't the one to go to, especially for newbies like me who think 0 Nh3/Nh4 and No2 means the tank is cycled, while it's not. Luckily I'm quite aware of the fact that there must be No3 trace in your water for the tank to be considered cycled. I had my suspicion that I could have bought a counterfeit one, but the date on the box is still 1 year away from its expiration date, so I went on to check the reviews on Amazon. I realised that a lot of people had the same problem. There're only two possibilities: Me having bought a counterfeit kit, or the Salifert test being unsuitable for reading a cycling tank, especially for newbies.I’m not saying the Salifert kit is correct, but how do you know the Sera is right and Salifert is wrong?
I’d personally ignore any ammonia values of 0.5 ppm or less.

