- Joined
- May 22, 2016
- Messages
- 6,565
- Reaction score
- 10,146
Ok. As a follow-up to adapting the Red Sea Nitrate test to be read by the Hanna Phosphorus ULR checker, lets spin the random wheel of Reef testing....
Nutrient: Iodide!
Test Kit: Seachem!
Colorimeter: Hanna Silica Low Range!
yeah, I know. A nutrient no one measures checked with a colorimeter no one has.
Anyway...
Take 10 ml of sample water in the hanna cuvette. Pour the 1 scoop of Iodide reagent 1 powder into the cuvette, shake it for like 20-30sec (it won't dissolve). This is your blank "c1" in the hanna meter.
Instead of using two drops of Iodide reagent 2, you'll drop twenty into the cuvette, because the sample is way bigger than intended.
Add drops quickly and time yourself. shake for a couple seconds it'll go blue- this is your "c2". Assuming its a low amount of color (Iodide <0.02) , run the "c2" between 70 seconds and 2 minutes after you started drops.
If it's a lot of color (Iodide ~.06ppm) then run "c2" 40 to 70 seconds after you started drops. The color dissipates fast after that.
...then convert...
Iodide ppm = 0.02997*(hanna reading) + 0.00104
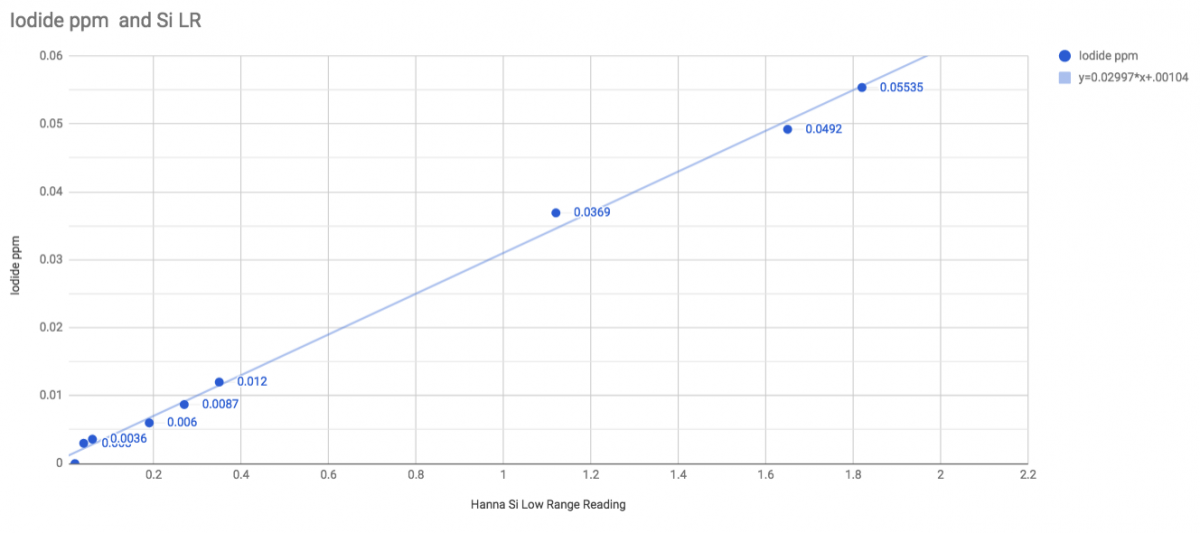
This will work for Iodide below 0.06ppm (50/50 chance it'll max the meter at 0.06), down to about 0.003ppm Iodide. Below that, you're within margin of error of zero.
For context, Seachem says natural sea water levels are 0.06ppm Iodide, and recommends dosing to that target.
Anyway, this would allow you to detect Iodine down to 1/20th of their recommended target value.
In theory one could use this to plot the Iodine depletion rate from 0.06ppm down to undetectable.
Nutrient: Iodide!
Test Kit: Seachem!
Colorimeter: Hanna Silica Low Range!
yeah, I know. A nutrient no one measures checked with a colorimeter no one has.
Anyway...
Take 10 ml of sample water in the hanna cuvette. Pour the 1 scoop of Iodide reagent 1 powder into the cuvette, shake it for like 20-30sec (it won't dissolve). This is your blank "c1" in the hanna meter.
Instead of using two drops of Iodide reagent 2, you'll drop twenty into the cuvette, because the sample is way bigger than intended.
Add drops quickly and time yourself. shake for a couple seconds it'll go blue- this is your "c2". Assuming its a low amount of color (Iodide <0.02) , run the "c2" between 70 seconds and 2 minutes after you started drops.
If it's a lot of color (Iodide ~.06ppm) then run "c2" 40 to 70 seconds after you started drops. The color dissipates fast after that.
...then convert...
Iodide ppm = 0.02997*(hanna reading) + 0.00104
This will work for Iodide below 0.06ppm (50/50 chance it'll max the meter at 0.06), down to about 0.003ppm Iodide. Below that, you're within margin of error of zero.
For context, Seachem says natural sea water levels are 0.06ppm Iodide, and recommends dosing to that target.
Anyway, this would allow you to detect Iodine down to 1/20th of their recommended target value.
In theory one could use this to plot the Iodine depletion rate from 0.06ppm down to undetectable.



















