I have seen this once - a half a year ago - but newer after that - here is the latest additions logged against the pH curve
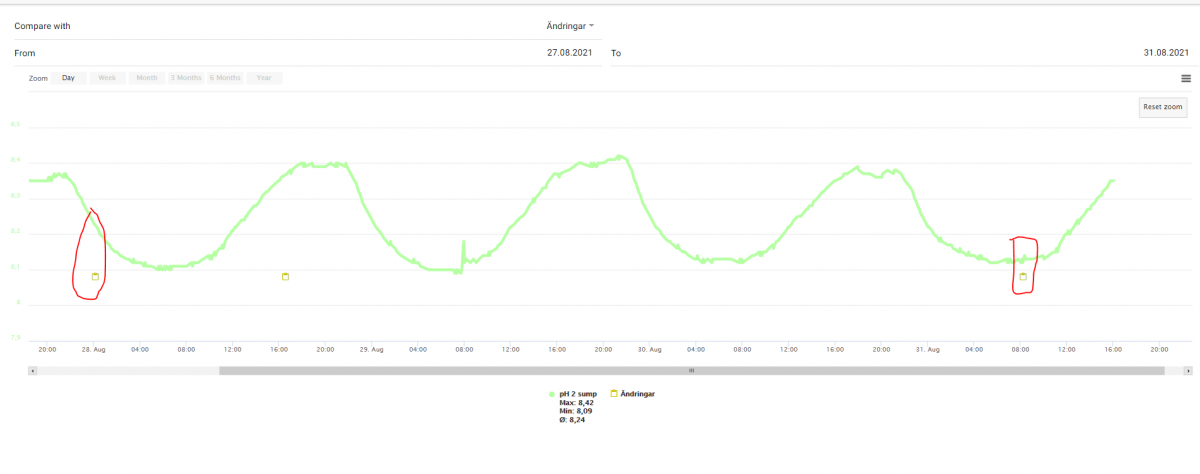
Sincerely Lasse
My experiment consisted of filling a liter beaker to the 1000ml mark with fresh mixed TM. Then testing for PH and then adding some 3% supermarket H2O2 and then retest for PH. I forget the exact amount of PH rise, but it was significant enough to cause me to scratch my head and do some research.
FWIW



















