- Joined
- May 22, 2016
- Messages
- 6,594
- Reaction score
- 10,186
did you mean to flip those? Red in the pic = h2o2 first, Blue = potato after?blue= addition of total 5 ml H2O2; Red = addition of 2 g chopped (fine) potatoe
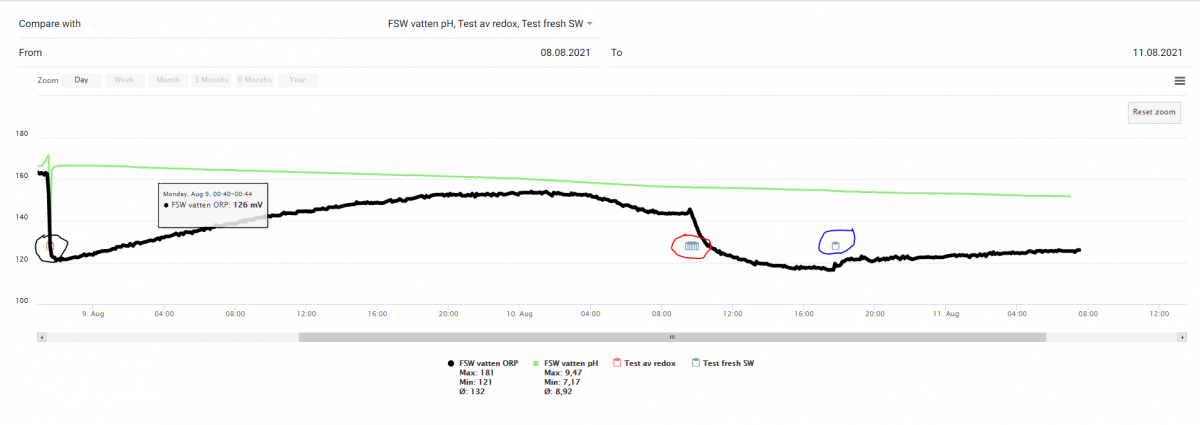
The addition of H2O2 did cause a ORP drop - but not so dramatically. Addition of a chopped potato did cause an ORP increase - but not very much.





















