I've used two types of Ocean Optics spectrometers to look at various lamps' spectra and have found these closely match those provided by the manufacturers.I have a hard time believing that light spectra from LED's are any more continuous that those from MH's or T5's. From wikipedia:
A light-emitting diode (LED) is a semiconductor light source that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor.[5] White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device.[6]
The band gap is an energetic transition of fixed and uniform magnitude (depending upon the materials used of course). While there is a small amount of spread in the wavelengths emitted from this band gap it's mostly monochromatic. And in the case of white LED's a phosphor is often used - this would act very similarly to a fluorescent lamp that uses a mixture of phosphors coated onto the inside of the tube that each emit monochromatically upon excitation with UV light. The greater the variety of phosphors used the closer the net emissions may approach a continuous spectrum, but it will never be anything more than a facsimile of a continuum.
None of the light sources we use over our tanks emit a true continuum as does the sun or black body radiation in general. That would require a filament lamp where the filament literally gets as hot as 10,000-20,000 Kelvin degrees! haha (and a gigantic tank cooler too!)
I suspect any spectragraphs provided by the manufacturers of LED's or T5 or MH's that show what appears to be a continuum are greatly smoothing that graph (i.e. lying). since the mechanism by which these devices produce light is by its nature discrete.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Distorted/mutant morphology in Acroporas radial and axial corallite structure.
- Thread starter C. Eymann
- Start date
- Tagged users None
I've used two types of Ocean Optics spectrometers to look at various lamps' spectra and have found these closely match those provided by the manufacturers.
That's really interesting... Why type of lamps are you referring to here? T5, MH, LED?
I bought the specs in 2002 and have tested all types of fluorescent lamps (Power Compacts, T8s, T5s), metal halides, mercury vapors, and LEDs. I've posted some spectral graphs here on R2R and quite a few on Advanced Aquarist. I've never seen any manufacturers' graphs that gave me heartburn but have seen exaggerated claims about the spectrum being 'best' for growing corals.That's really interesting... Why type of lamps are you referring to here? T5, MH, LED?
The only spectra that make me suspicious are those of the LED's... that they depict such a continuous and smooth curve (T5 and MH spectra have all the discontinuous emission spikes I would expect to see).
How is it that the LED's are producing such a smooth continuum? Or have the manufacturers of the modern devices gone far beyond the basic technology explained by wikipedia?
How is it that the LED's are producing such a smooth continuum? Or have the manufacturers of the modern devices gone far beyond the basic technology explained by wikipedia?
very good OP and made me think about some things, I have had my experience with the "popular" LED lighting and notice alot of things in alot species thats off with them . So I am trying the newest LED tech that the tech made sense to me If I was gonna build it , I would mimic our beloved Radiums that provided the best health ,coloration ,growth for me, and yes I thought I was crazy with some of the bumpy A.tenuis I have seen lately thought I was getting old and my eyes going lol (which is prob true too) So tommorow , I am going to mount a frag of Pulp Fiction in the DT and document it under these new lights and see if this is an issue for me on my new setup. Red planet / Bonzi for example are really growing like MAD and with perfect structure so far. But you are saying you see it in A. Tenuis examples right , nothing else ?
very good OP and made me think about some things, I have had my experience with the "popular" LED lighting and notice alot of things in alot species thats off with them . So I am trying the newest LED tech that the tech made sense to me If I was gonna build it , I would mimic our beloved Radiums that provided the best health ,coloration ,growth for me, and yes I thought I was crazy with some of the bumpy A.tenuis I have seen lately thought I was getting old and my eyes going lol (which is prob true too) So tommorow , I am going to mount a frag of Pulp Fiction in the DT and document it under these new lights and see if this is an issue for me on my new setup. Red planet / Bonzi for example are really growing like MAD and with perfect structure so far. But you are saying you see it in A. Tenuis examples right , nothing else ?
only A. Tenuis for me.
Here's the spectral signature of a LED with all 9 channels at full power (390nm, 423nm, 447nm, 462nm, 545nm, 594nm, and 3 producing broad spectra ('white.')
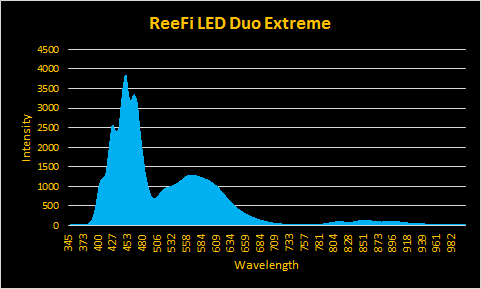
Here's the spectral signature of a LED with all 9 channels at full power (390nm, 423nm, 447nm, 462nm, 545nm, 594nm, and 3 producing broad spectra ('white.')

Well I'll be darned... Dana you've proven me wrong. Apparently the spread of the emission peaks from LEDs is wider than I gave it credit for. I'll have to do some reading into why this is the case.
Why do you think the manufacturer left a gap right there at 506nm? Seems like that would be prime territory for reef light? Perhaps a materials limitation? Or is that by design?
That coral is from another thread that is about to die.I guess I should post a pic or two for my example
Top coral is a A. tenuis that has been maricultured (propagated and grown out in the ocean under natural sunlight)
Notice how the radial corallites rims' are smooth an fairly uniform.

Here is a tenuis grown under LEDs that a user posted just recently.
Notice the irregular/ rough distorted structure of the radial corallites.

These are my “Healthy” led only corals
I'll be the first to admit I'm no Acropora expert. In fact, my one and only Acro is just now starting to take off again after existing but not thriving for quite some time. However, even under Kessil LEDs, I see no anomalous growth abnormalities (for what that's worth):
![IMG_20191112_200238[1].jpg IMG_20191112_200238[1].jpg](https://www.reef2reef.com/attachments/img_20191112_200238-1-jpg.1283848/)
BTW (not to hijack the thread), but what the heck is that in the top middle of the last picture? (no, NOT the coral)That coral is from another thread that is about to die.
These are my “Healthy” led only corals




I guess they must dope the semiconductors to get a broader emission spectrum, probably through closely-spaced, overlapping peaks.Here's the spectral signature of a LED with all 9 channels at full power (390nm, 423nm, 447nm, 462nm, 545nm, 594nm, and 3 producing broad spectra ('white.')

I did not mean for this thread to turn into a LED vs the world debate, I'm sorry if it was interpreted that way, totally not the case, I dont find these distortions bad or undesirable, on the contrary I find it very interesting, I wanted to know more about why I am seeing these distortions
From my experience, I have never seen these distortions until people started growing acropora under LED only about 8 or 9 years ago, I have noticed it much more prevalent in LED only vs hybrid systems, or T5/Halide/sunlight only.
Thank you @Dana Riddle for you contribution of knowledge.
I apologize to everyone that this started a dead horse LED debate.
Again, I'm not viewing these distortions as problematic or undesirable, I think its actually pretty neat! I was just simply asking if other stick heads noticed and if they knew possible reasons.
That is all
From my experience, I have never seen these distortions until people started growing acropora under LED only about 8 or 9 years ago, I have noticed it much more prevalent in LED only vs hybrid systems, or T5/Halide/sunlight only.
Thank you @Dana Riddle for you contribution of knowledge.
I apologize to everyone that this started a dead horse LED debate.
Again, I'm not viewing these distortions as problematic or undesirable, I think its actually pretty neat! I was just simply asking if other stick heads noticed and if they knew possible reasons.
That is all
I didn't interpret it that way at all! In fact, I believe I learned something myself, and I appreciate all of those willing to discuss the topic without random, ad-homsI did not mean for this thread to turn into a LED vs the world debate, I'm sorry if it was interpreted that way, totally not the case, I dont find these distortions bad or undesirable, on the contrary I find it very interesting, I wanted to know more about why I am seeing these distortions
From my experience, I have never seen these distortions until people started growing acropora under LED only about 8 or 9 years ago, I have noticed it much more prevalent in LED only vs hybrid systems, or T5/Halide/sunlight only.
Thank you @Dana Riddle for you contribution of knowledge.
I apologize to everyone that this started a dead horse LED debate.
Dragon faced pipefishBTW (not to hijack the thread), but what the heck is that in the top middle of the last picture? (no, NOT the coral)
Oh, lawd!! I figured it must be something like thatDragon faced pipefish
Dragon faced pipefish
Pipefish are way cool, supposedly they are great at potential acropora pest control too! but need fairly gentle currents so I never felt comfortable keeping them.
I love these little guys. They just snake around the rocks and pic at pods all day and night. I don’t have the crazy sps flow that some use but my lfs store keeps a pair in their tank and the flow is insane. Doesn’t bother the pipefish at all. I will say they are the largest pipefish I’ve ever seen though.Pipefish are way cool, supposedly they are great at potential acropora pest control too! but need fairly gentle currents so I never felt comfortable keeping them.
Pipefish. Super coolBTW (not to hijack the thread), but what the heck is that in the top middle of the last picture? (no, NOT the coral)
Similar threads
- Replies
- 20
- Views
- 921
- Replies
- 225
- Views
- 7,259
- Replies
- 5
- Views
- 706
- Replies
- 2
- Views
- 377
TOP 10 Trending Threads
- Replies
- 39
- Views
- 606
- Replies
- 27
- Views
- 476
-
- Sticky
- Replies
- 113
- Views
- 842
- Replies
- 60
- Views
- 1,428
- Replies
- 29
- Views
- 808
- Replies
- 37
- Views
- 942
- Replies
- 21
- Views
- 255
- Replies
- 24
- Views
- 352
New Posts
-
Black Friday HOT DEALS SBB - 500 Auctions Up! Starting at $1
- Latest: armyvetheather
-
-
-
West Virginia Live Goods Sps 13 Pack (HW,WD,Fury,Loom & others)
- Latest: WVReefJunkie