In a previous article I discussed my thoughts on trace elements

 www.reef2reef.com
www.reef2reef.com
This article expands on that discussion by providing specific guidance for all elements one might dose.
First, some explanation.
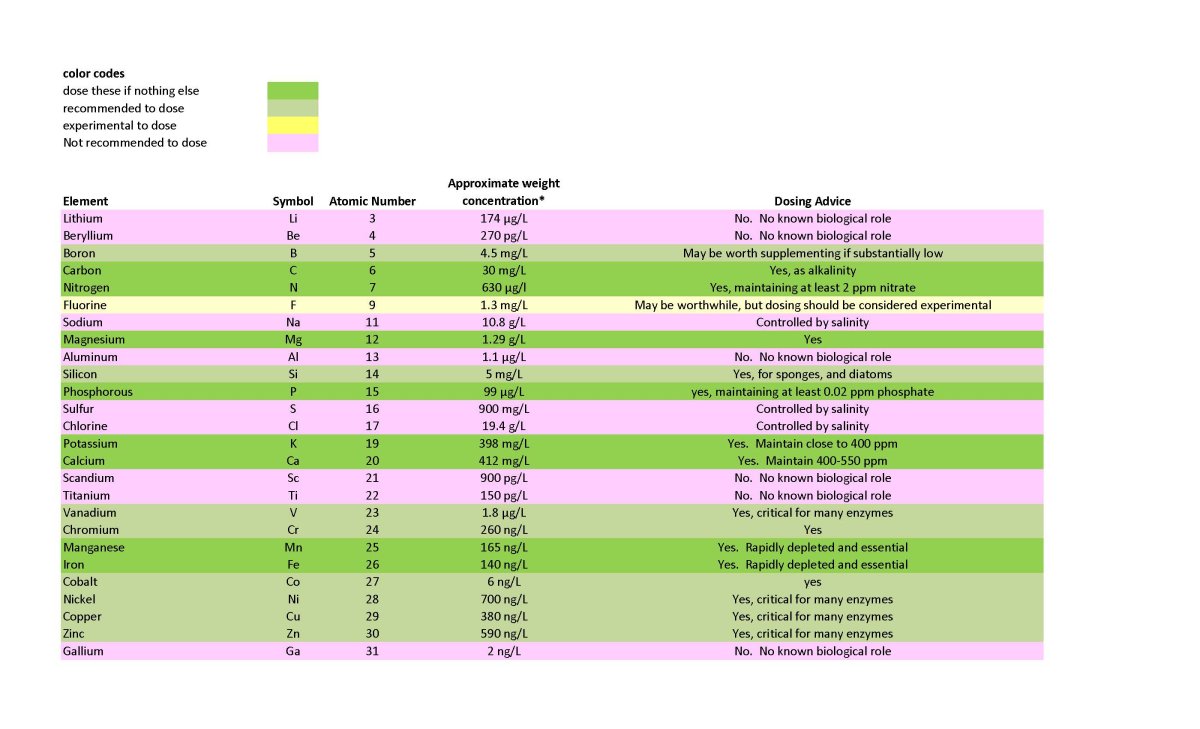
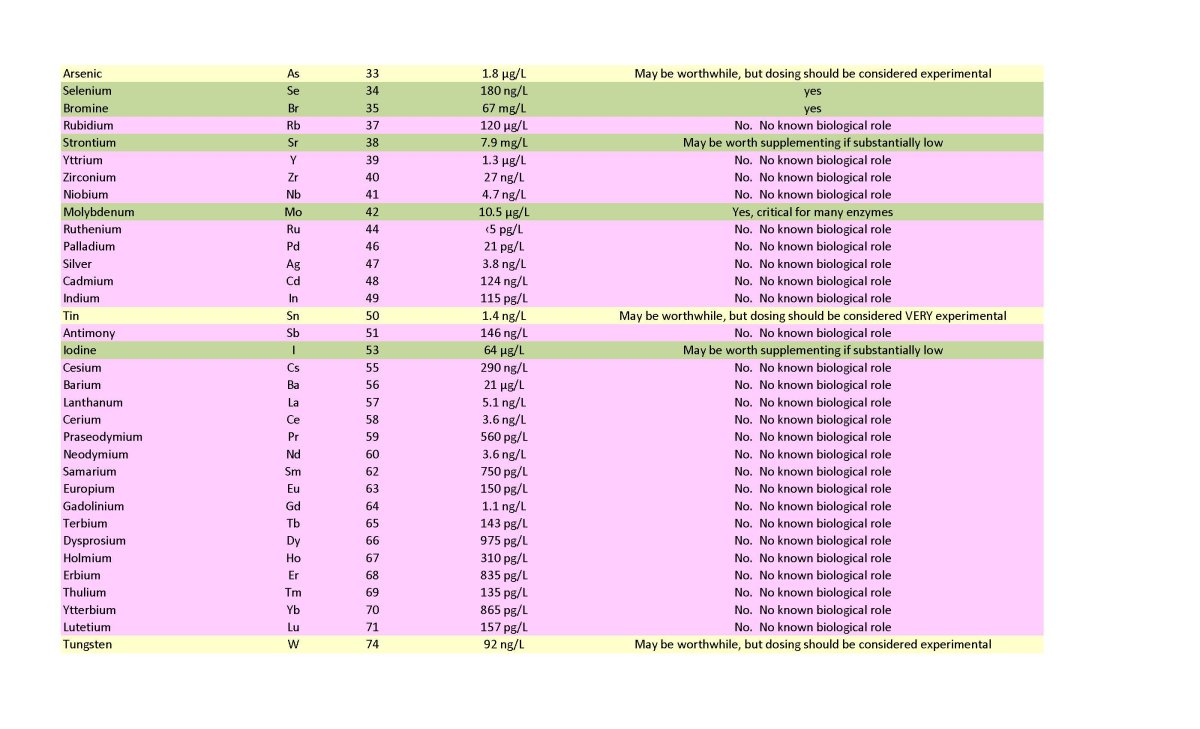
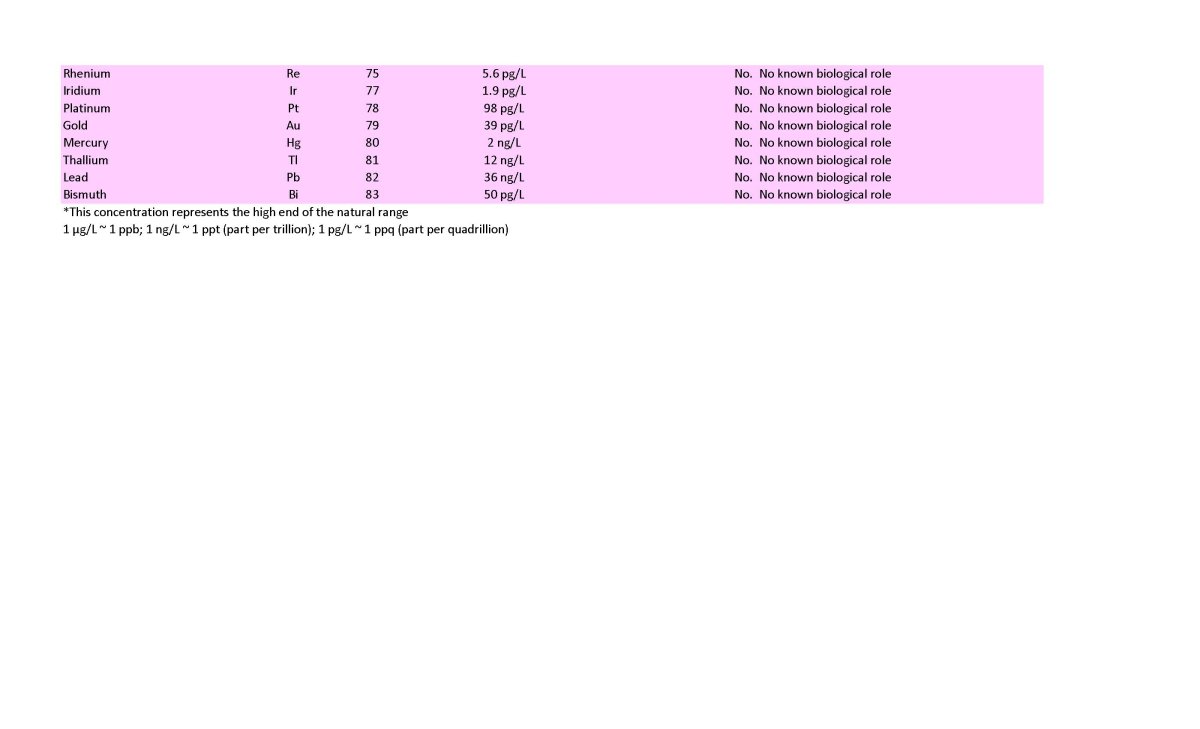
I have listed many elements as not recommended to dose based on the lack of evidence of a requirement in ANY known organism. These are shown in a lavender color on the table below. I see no rationale to dose these, despite many being at odds with recommendations that permeate the reefing community. In many cases those recommendations may stem from the false assumption that if a coral skeleton deposits an element, it must be useful for the coral. That assumption is just not true. Precipitating calcium carbonate incorporates all sorts of elements from seawater that have no benefit, up to and including uranium. Barium seems to fit that idea, and I reject the idea that it is needed or useful. Also colored in lavender are a few elements that are only known to be used by a single microorganism that is unlikely to be important in any reef tank, and they drop off the dosing list. Cadmium is such an example.
Second, just because I recommend an element DOES NOT mean I agree with the wide ranging claims for those elements. I do not believe strontium is useful for hard corals, for example, but it does have uses in other organisms and makes the dosing list. Likewise, I do not believe shrimp need iodine, but it makes the dosing list because it is used by other organisms such as some gorgonia.
Third, some elements may be required by some or even many organisms but also may have the potential for toxicity if overdosed and one needs to be very careful dosing these. These elements include copper and arsenic.
Finally, some elements lack much info on what happens if they are not maintained at all, and these I have put in the experiment category, even if there are organisms somewhere known to use then. Fluoride, arsenic, tin, and tungsten fit this category.
Recommendations
Before getting to anything else, note that a yes for dosing in the table below does not mean it needs to be dosed in all tanks. It means that it may be needed if levels are sufficiently low. I do not recommend individual random dosing without measurement except for iron, manganese and silicate that are all rapidly depleted and show little harm from overdosing. That said, dosing certain commercial trace element mixes without measurement may be acceptable depending on the system and the reliability of the company providing the solution and dosing guidelines.
The table below recommends dosing a variety of major ions as needed (e.g., potassium, calcium, magnesium, etc.), but those are not the point of this article. Two elements (N as nitrate and P as phosphate) also make the list to maintain, and sometimes are considered trace elements, but are not the main focus of this article.
Two trace elements warrant special attention since they rapidly deplete and are used by a great many organisms: iron and manganese. If you dose no other trace elements, these two would be the two I'd pick.
I recommend silicate dosing if you care about either sponges, or the ability of diatoms to displace more problematic pests from surfaces such as rocks.
There are a number of other trace elements that are very important for organisms (e.g., vanadium). Whether these get low enough to warrant dosing may be system dependent, and reefers lack good info on what levels are too low for any given organism we keep, but it is not hard to maintain detectable levels and/or look for biological effects when dosing.
I'm sure many folks will be disappointed that I am not providing a specific concentration target or range for each element. The reason I do not, even when others do so, is because I do not think there is sufficient data to make such recommendations. Even recommending natural levels is fraught with problems. This is why:
1. Accurate measurement of trace elements is not assured even by using and type of ICP. Proper procedures need to be followed, and we rarely have such info.
2. The chemical form present in an aquarium greatly impacts it bioavailability and toxicity. These chemical forms can include oxidations tates 9e.g., iodide and iodate for iodine) and in the binding of the elements by organic matter (e.g., copper is known to be nearly completely bound by organics in the ocean, significantly reducing its toxicity realtive to bare copper ion.
3. Reef hobbyists keep a very wide range of organisms. if someone suggests a particular target concentration for a trace element, what organism is that based on? Do you even keep that organism?
4. Even knowing the chemical form of trace element in a reef tank (we do not) and somehow matching it to the ocean concentration (where and at what depth), the ocean itself may not be optimal. Iron is known to be limiting to the growth of some organisms in the ocean. Do we want to have that same limitation, or not?
To this end, I provide the whole ocean average concentration in the table below. Trace elements vary by depth and by location. Iron and silicate, for example, are greatly depleted in surface water relative to deeper water because of organisms grabbing them up as fast as they can. That said, ocean concentrations can be at least a very rough guide to whether trace elements measured by ICP may be too low or too high.
All of these thoughts are just my opinion, and I recognize that others have divergent opinions on what should be done about trace elements. I welcome any discussions, corrections, clarifications, or differences of opinion in the thread that is associated with this article.
Since the table may not read well on all devices, i have attached the table as a pdf as well.
Happy Reefing!

Randy's thoughts on trace elements
Randy's thoughts on trace elements in reef aquaria.
 www.reef2reef.com
www.reef2reef.com
This article expands on that discussion by providing specific guidance for all elements one might dose.
First, some explanation.
I have listed many elements as not recommended to dose based on the lack of evidence of a requirement in ANY known organism. These are shown in a lavender color on the table below. I see no rationale to dose these, despite many being at odds with recommendations that permeate the reefing community. In many cases those recommendations may stem from the false assumption that if a coral skeleton deposits an element, it must be useful for the coral. That assumption is just not true. Precipitating calcium carbonate incorporates all sorts of elements from seawater that have no benefit, up to and including uranium. Barium seems to fit that idea, and I reject the idea that it is needed or useful. Also colored in lavender are a few elements that are only known to be used by a single microorganism that is unlikely to be important in any reef tank, and they drop off the dosing list. Cadmium is such an example.
Second, just because I recommend an element DOES NOT mean I agree with the wide ranging claims for those elements. I do not believe strontium is useful for hard corals, for example, but it does have uses in other organisms and makes the dosing list. Likewise, I do not believe shrimp need iodine, but it makes the dosing list because it is used by other organisms such as some gorgonia.
Third, some elements may be required by some or even many organisms but also may have the potential for toxicity if overdosed and one needs to be very careful dosing these. These elements include copper and arsenic.
Finally, some elements lack much info on what happens if they are not maintained at all, and these I have put in the experiment category, even if there are organisms somewhere known to use then. Fluoride, arsenic, tin, and tungsten fit this category.
Recommendations
Before getting to anything else, note that a yes for dosing in the table below does not mean it needs to be dosed in all tanks. It means that it may be needed if levels are sufficiently low. I do not recommend individual random dosing without measurement except for iron, manganese and silicate that are all rapidly depleted and show little harm from overdosing. That said, dosing certain commercial trace element mixes without measurement may be acceptable depending on the system and the reliability of the company providing the solution and dosing guidelines.
The table below recommends dosing a variety of major ions as needed (e.g., potassium, calcium, magnesium, etc.), but those are not the point of this article. Two elements (N as nitrate and P as phosphate) also make the list to maintain, and sometimes are considered trace elements, but are not the main focus of this article.
Two trace elements warrant special attention since they rapidly deplete and are used by a great many organisms: iron and manganese. If you dose no other trace elements, these two would be the two I'd pick.
I recommend silicate dosing if you care about either sponges, or the ability of diatoms to displace more problematic pests from surfaces such as rocks.
There are a number of other trace elements that are very important for organisms (e.g., vanadium). Whether these get low enough to warrant dosing may be system dependent, and reefers lack good info on what levels are too low for any given organism we keep, but it is not hard to maintain detectable levels and/or look for biological effects when dosing.
I'm sure many folks will be disappointed that I am not providing a specific concentration target or range for each element. The reason I do not, even when others do so, is because I do not think there is sufficient data to make such recommendations. Even recommending natural levels is fraught with problems. This is why:
1. Accurate measurement of trace elements is not assured even by using and type of ICP. Proper procedures need to be followed, and we rarely have such info.
2. The chemical form present in an aquarium greatly impacts it bioavailability and toxicity. These chemical forms can include oxidations tates 9e.g., iodide and iodate for iodine) and in the binding of the elements by organic matter (e.g., copper is known to be nearly completely bound by organics in the ocean, significantly reducing its toxicity realtive to bare copper ion.
3. Reef hobbyists keep a very wide range of organisms. if someone suggests a particular target concentration for a trace element, what organism is that based on? Do you even keep that organism?
4. Even knowing the chemical form of trace element in a reef tank (we do not) and somehow matching it to the ocean concentration (where and at what depth), the ocean itself may not be optimal. Iron is known to be limiting to the growth of some organisms in the ocean. Do we want to have that same limitation, or not?
To this end, I provide the whole ocean average concentration in the table below. Trace elements vary by depth and by location. Iron and silicate, for example, are greatly depleted in surface water relative to deeper water because of organisms grabbing them up as fast as they can. That said, ocean concentrations can be at least a very rough guide to whether trace elements measured by ICP may be too low or too high.
All of these thoughts are just my opinion, and I recognize that others have divergent opinions on what should be done about trace elements. I welcome any discussions, corrections, clarifications, or differences of opinion in the thread that is associated with this article.
Since the table may not read well on all devices, i have attached the table as a pdf as well.
Happy Reefing!











