- Joined
- Feb 22, 2018
- Messages
- 1,289
- Reaction score
- 607
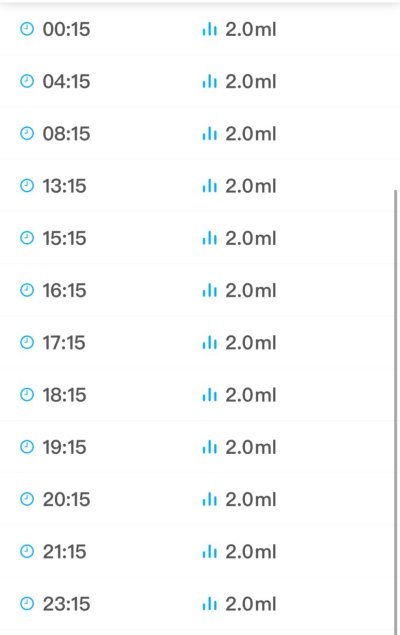
are you dosing .1 ppm x4 or spread out .1 through 4 times , I putted it into my ato , think that’s okay ?I've been dosing 0.4ppm of ammonia (ammonium carbonate stock solution as described by Randy, 0.1ppm four times per day) and so far so good. I'm still getting zero nitrates but growth has resumed and maxima's mantles more expanded.