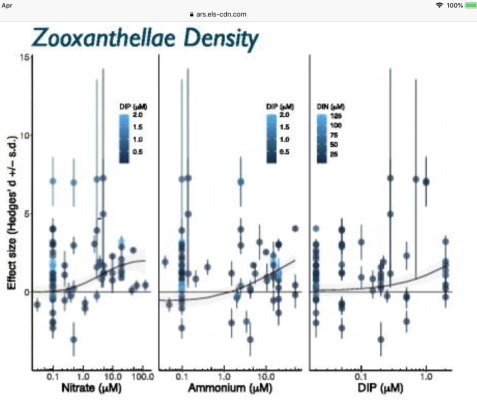
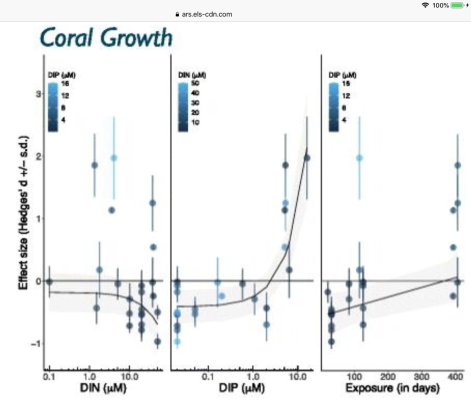
Nitrate is tough think to "eat" it needs a lot of energy to be reduced back to ammonia. There is no way to "incorporate" nitrate into an amino acid directly, nitrogen should be in more reduced form for this. The process of nitrate assimilation is almost the same for algae (micro and macro), plants and some bacteria.Thanks. I had not read that macro used high energy to convert no3, but I believe you and will add this to my calculus. BTW - we adopted 2 girls from Smolyan... I did not see too many reef tanks there in the mountains.
My best regards to the girls