I have no disagreement. And - the products I'm talking about are mostly FW - but say also SWIt’s a cold cruel world filled with false claims. It’s seriously stomach churning sometimes.
In my opinion, the claims on nitrate toxicity reduction in marine systems is nonsense. I go into lengthy detail to justify that opinion of mine here:

Reefers may over-rely on personal experience to accept or reject truth
Really? You didn't know from somewhere that corals need light? calcium? Alkalinity? sources of nutrients? It may sound ridiculous, but some of those were hard won bits of information determined by the failures of early reefers. I'm sorry. You are correct. I was looking beyond the basics when...www.reef2reef.com
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Does Prime actually "Detoxify" free ammonia, NH3?
- Thread starter taricha
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,625
- Reaction score
- 10,228
I did the same (but much lower tech than Dan) he got better films and analyzed them in a more sophisticated way. But here's what a hobbyist trying to measure ammonia reduction with ClorAm-X will find.I worked with ClorAmX after reading the patent. I was optimistic about seeing an effect. I could not reproduce the patent observations with three different ammonia sensing films and a spectrophotometer. Also note the large concentrations being used in the patent versus the recommended dosage.
100 mL tank water samples, on orbital shaker with 2ppm total ammonia and ClorAm-X overnight....
pulled out to photograph, pH 7.6. left is no clmx, right with clmx recommended for 2ppm
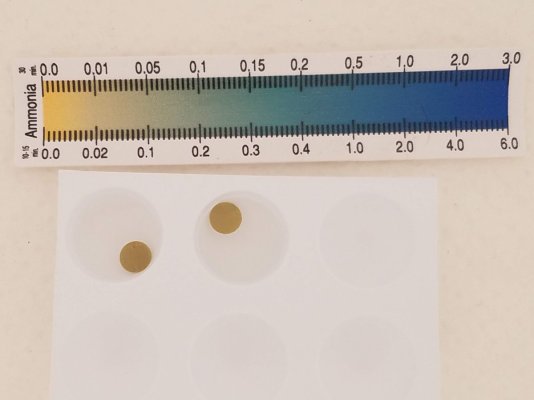
bump pH to 8.3, add another disk to each - 3 hours on shaker later, left is (-clmx), right (+clmx)
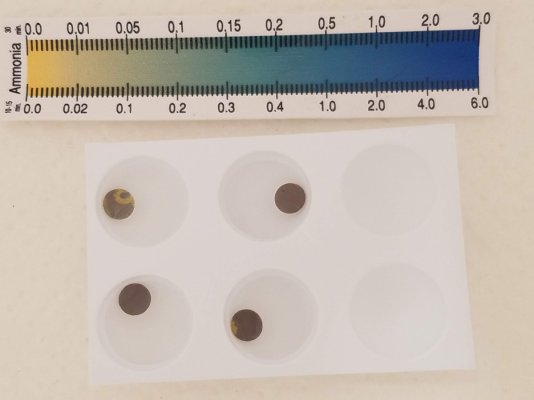
drop pH to 8.0, add another disk to each - photo is 4 hours of shaking later. top is (-clmx), bottom (+clmx)

So to the extent hobbyists can observe, addind ClorAm-X doesn't decrease NH3/4 or bind it into anything that doesn't simply obey the same pH relationship as NH3/4.
By the time of the last pic, it'd been 24 hours. Biospira or Fritz would have totally cleared the 2ppm ammonia in that time frame.
So let me go through the ClorAm-X patent (next post).FWIW, Kuhns does provide efficacy data in his patent:
https://patentimages.storage.googleapis.com/d3/5e/a2/8a6cbe50706255/EP0203741B1.pdf
Last edited:
- Joined
- May 22, 2016
- Messages
- 6,625
- Reaction score
- 10,228
FWIW, Kuhns does provide efficacy data in his patent:
Quickly(?), here's a few reasons why the patent doesn't persuade me that ClorAm-X etc would protect from ammonia in a hobby saltwater setting. (It may, but what's presented isn't convincing at all.)
Looking at the example experiments in the patent:
Example 1 is a freshwater test using 10 Normal NaOH to push the pH of the ammonia chloride solution so high (not measured) that presumably ~100% of the ammonia is NH3, so it can all be measured with the ion selective electrode. (The electrode doesn’t measure total ammonia, NH3+NH4, it measures NH3 only).
The amount of NH3 is 0.5 to 5ppm free ammonia and the pH is who-knows-how high. Reef hobbyists want to handle stuff more in the ballpark of ~0.1ppm NH3 at pH 8.
But even at very high NH3 and very high pH, the product only reduced 0.5ppm NH3 by 10%. The % reductions got better at very high NH3, and worse at the "low" concentrations, suggesting that in our ballpark of ~0.1ppm NH3 and below, the removal might be unobservable in a real saltwater system.
Examples 2, 3, and 4 are test tube reactions directly between high concentrations of ammonium hydroxide and the product. Would the same reactions occur at single digit ppm concentrations in saltwater?
Example 5 is about the dechlorinating property, which it verifiably does.
Example 6 is observation that it doesn't make any weird reactions by adding the product to fresh or saltwater.
No Example 7
Example 8 is that the product itself is not toxic to freshwater fish in high doses.
Example 9 shows that it protects freshwater fish from chlorinated tap water.
Example 10 shows that overdoses of the product don't hurt condy anemones in saltwater.
Example 11 was a freshwater test involving a large number of fish. It seems that the only toxic condition that the fish were subjected to was water changes with tap water, and if I read it right, a normal dechlorinator and ClorAm-x both kept the fish alive (with statistically insignificant exceptions).
Example 12 is saltwater data at some aquarium-relevant ammonia and pH levels that looks like it shows reduction of ammonia. So let’s take a minute and unpack, since it's the most relevant.
First, in one part the experimenter is trying to measure tiny amounts of free ammonia (total ammonia = 1.0, pH 6.0, so free ammonia is 0.00032 ppm NH3 ). Ion-Selective Electrodes like this from Hach (or orion) don’t go near that low - only to 0.01mg/L NH3. To get around the fact that the starting concentration is 1/20th of the normal lower range of these meter and what’s wanted is to measure reduction below that…. here’s what was done: mix the concentration to 1.00ppm TOTAL ammonia, recalibrate the Ion Selective Electrode to tell the meter that it is actually 1.00ppm FREE ammonia (NH3) and then track the reduction with the meter from that point on. This is reported as relative decrease in ammonia. So table 5 is saying that the electrode measured a 32% decrease in free ammonia NH3 from 0.00032 mg/L to 0.00022mg/L. Seems that detection of a decrease of 1 tenth of a part per billion NH3 would be really hard to replicate.
I don’t trust that just calibrating the meter at way below its lower detection limit gets around the lower limits of such devices, but that’s what was done.
Let's just assume for now that the calibration method worked and the meter gave real values, maybe it did. The more fundamental issue is that no mention is made of how pH of saltwater was set to 6.0, 7.0, 8.0, and 9.0 for the various tests, and no mention is made of any attempt to control or measure pH during the application of the product.
This is fundamental because when I measure pH of adding ClorAm-X to saltwater, it drops the pH by a tenth at low doses and several tenths at high doses (with or without ammonia present). @Dan_P also measured a similar pH drop. So did this paper, that needed to buffer the constant low pH while using ClorAm-X in intensive culture.
“ClorAm-X is an alkali metal formaldehydebisulfite that binds to unionized ammonia creating an aminomethanesulfonate salt and thereby reducing TAN. This molecule is nontoxic and does not interfere with the nitrogen cycle (Kuhns 1987). The neutralizing of free ammonia releases a hydrogen ion, lowering pH. To buffer the system, 5 g of sodium bicarbonate/d was added to the ClorAm-X solution”
To see why the pH is a thing to be nitpicky about, take the data from the most applicable situation for the reef hobbyist - Table 11 (Ion Selective Electrode is well within its range here).
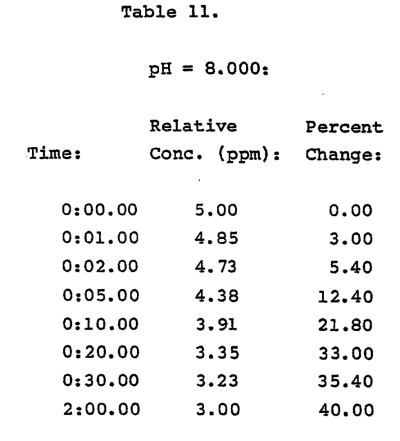
Saltwater at 1.020 s.g. was set to total ammonia of 5.0ppm and pH of 8.0. The reduction in measured free ammonia (NH3) that was achieved after 2 hrs was 40%. It had basically leveled off by 30 minutes, with almost no change from 30 min to 2 hours.
I took my tank water, diluted to 1.020s.g. and added 5ppm total ammonia, and measured the pH with ClorAm-X addition. It was initially stable at 7.89 pH, and when adding ClorAm-X, it dropped to 7.74 pH by 2 hours with most of the initial drop recorded in the first ~10 minutes. Running these pH values into the calculator, predicts a reduced NH3 from 0.1272 to 0.0907 or ~30% just from pH. Comparing to table 11’s measured 40% reduction, it’s hard to see what it is that ClorAm-X is supposed to have done to the ammonia. It looks like the “reaction” documented in the most relevant data may have just been a pH drop from ClorAm-X and maybe little else.
But even if it were a completely real NH3-binding reaction and pH were controlled, the data says that reduction of NH3 in a saltwater tank is limited and never actually goes low enough to be acceptable (5ppm to 3ppm total ammonia).
Example 13 shows that they took marine livestock, shipped them in bags from the Virgin Islands to Kansas City over 48hrs. The product was added to every bag and the animals survived. No attempt was made to ship some without the product to show a difference and no measurements of any kind (pH, NH3) were done.
Example 14 is the same exercise as example 12, but in hard freshwater instead of saltwater.
Example 15 is much the same as example 12, but used solid product instead of dissolved. Again, 1ppm total ammonia at pH 6.8 is a very tiny free ammonia amount (0.002 mg/L NH3) and is below detection limit for the NH3 Ion Selective Electrodes that I can find from Hach and Orion.
And that’s all the example data in the patent. And I see nothing that persuades me that it reacts to neutralize ammonia in saltwater in any significant degree, nor was any attempt made to demonstrate that saltwater livestock fared better with the product than without.
So big picture - why bother with something that may do nothing but lower pH a tenth or two? especially when you consider that you can dump in a bottle of Fritz Turbo Start and go from 8ppm to zero in under a day, and Biospira in high doses can be nearly as fast - there’s really no reason I can see to rely on hydroxymethanesulfonate to address elevated ammonia.
- Joined
- Sep 21, 2018
- Messages
- 6,783
- Reaction score
- 7,262
Patents for chemical syntheses are similarly frustrating.Quickly(?), here's a few reasons why the patent doesn't persuade me that ClorAm-X etc would protect from ammonia in a hobby saltwater setting. (It may, but what's presented isn't convincing at all.)
Looking at the example experiments in the patent:
Example 1 is a freshwater test using 10 Normal NaOH to push the pH of the ammonia chloride solution so high (not measured) that presumably ~100% of the ammonia is NH3, so it can all be measured with the ion selective electrode. (The electrode doesn’t measure total ammonia, NH3+NH4, it measures NH3 only).
The amount of NH3 is 0.5 to 5ppm free ammonia and the pH is who-knows-how high. Reef hobbyists want to handle stuff more in the ballpark of ~0.1ppm NH3 at pH 8.
But even at very high NH3 and very high pH, the product only reduced 0.5ppm NH3 by 10%. The % reductions got better at very high NH3, and worse at the "low" concentrations, suggesting that in our ballpark of ~0.1ppm NH3 and below, the removal might be unobservable in a real saltwater system.
Examples 2, 3, and 4 are test tube reactions directly between high concentrations of ammonium hydroxide and the product. Would the same reactions occur at single digit ppm concentrations in saltwater?
Example 5 is about the dechlorinating property, which it verifiably does.
Example 6 is observation that it doesn't make any weird reactions by adding the product to fresh or saltwater.
No Example 7
Example 8 is that the product itself is not toxic to freshwater fish in high doses.
Example 9 shows that it protects freshwater fish from chlorinated tap water.
Example 10 shows that overdoses of the product don't hurt condy anemones in saltwater.
Example 11 was a freshwater test involving a large number of fish. It seems that the only toxic condition that the fish were subjected to was water changes with tap water, and if I read it right, a normal dechlorinator and ClorAm-x both kept the fish alive (with statistically insignificant exceptions).
Example 12 is saltwater data at some aquarium-relevant ammonia and pH levels that looks like it shows reduction of ammonia. So let’s take a minute and unpack, since it's the most relevant.
First, in one part the experimenter is trying to measure tiny amounts of free ammonia (total ammonia = 1.0, pH 6.0, so free ammonia is 0.00032 ppm NH3 ). Ion-Selective Electrodes like this from Hach (or orion) don’t go near that low - only to 0.01mg/L NH3. To get around the fact that the starting concentration is 1/20th of the normal lower range of these meter and what’s wanted is to measure reduction below that…. here’s what was done: mix the concentration to 1.00ppm TOTAL ammonia, recalibrate the Ion Selective Electrode to tell the meter that it is actually 1.00ppm FREE ammonia (NH3) and then track the reduction with the meter from that point on. This is reported as relative decrease in ammonia. So table 5 is saying that the electrode measured a 32% decrease in free ammonia NH3 from 0.00032 mg/L to 0.00022mg/L. Seems that detection of a decrease of 1 tenth of a part per billion NH3 would be really hard to replicate.
I don’t trust that just calibrating the meter at way below its lower detection limit gets around the lower limits of such devices, but that’s what was done.
Let's just assume for now that the calibration method worked and the meter gave real values, maybe it did. The more fundamental issue is that no mention is made of how pH of saltwater was set to 6.0, 7.0, 8.0, and 9.0 for the various tests, and no mention is made of any attempt to control or measure pH during the application of the product.
This is fundamental because when I measure pH of adding ClorAm-X to saltwater, it drops the pH by a tenth at low doses and several tenths at high doses (with or without ammonia present). @Dan_P also measured a similar pH drop. So did this paper, that needed to buffer the constant low pH while using ClorAm-X in intensive culture.
“ClorAm-X is an alkali metal formaldehydebisulfite that binds to unionized ammonia creating an aminomethanesulfonate salt and thereby reducing TAN. This molecule is nontoxic and does not interfere with the nitrogen cycle (Kuhns 1987). The neutralizing of free ammonia releases a hydrogen ion, lowering pH. To buffer the system, 5 g of sodium bicarbonate/d was added to the ClorAm-X solution”
To see why the pH is a thing to be nitpicky about, take the data from the most applicable situation for the reef hobbyist - Table 11 (Ion Selective Electrode is well within its range here).

Saltwater at 1.020 s.g. was set to total ammonia of 5.0ppm and pH of 8.0. The reduction in measured free ammonia (NH3) that was achieved after 2 hrs was 40%. It had basically leveled off by 30 minutes, with almost no change from 30 min to 2 hours.
I took my tank water, diluted to 1.020s.g. and added 5ppm total ammonia, and measured the pH with ClorAm-X addition. It was initially stable at 7.89 pH, and when adding ClorAm-X, it dropped to 7.74 pH by 2 hours with most of the initial drop recorded in the first ~10 minutes. Running these pH values into the calculator, predicts a reduced NH3 from 0.1272 to 0.0907 or ~30% just from pH. Comparing to table 11’s measured 40% reduction, it’s hard to see what it is that ClorAm-X is supposed to have done to the ammonia. It looks like the “reaction” documented in the most relevant data may have just been a pH drop from ClorAm-X and maybe little else.
But even if it were a completely real NH3-binding reaction and pH were controlled, the data says that reduction of NH3 in a saltwater tank is limited and never actually goes low enough to be acceptable (5ppm to 3ppm total ammonia).
Example 13 shows that they took marine livestock, shipped them in bags from the Virgin Islands to Kansas City over 48hrs. The product was added to every bag and the animals survived. No attempt was made to ship some without the product to show a difference and no measurements of any kind (pH, NH3) were done.
Example 14 is the same exercise as example 12, but in hard freshwater instead of saltwater.
Example 15 is much the same as example 12, but used solid product instead of dissolved. Again, 1ppm total ammonia at pH 6.8 is a very tiny free ammonia amount (0.002 mg/L NH3) and is below detection limit for the NH3 Ion Selective Electrodes that I can find from Hach and Orion.
And that’s all the example data in the patent. And I see nothing that persuades me that it reacts to neutralize ammonia in saltwater in any significant degree, nor was any attempt made to demonstrate that saltwater livestock fared better with the product than without.
So big picture - why bother with something that may do nothing but lower pH a tenth or two? especially when you consider that you can dump in a bottle of Fritz Turbo Start and go from 8ppm to zero in under a day, and Biospira in high doses can be nearly as fast - there’s really no reason I can see to rely on hydroxymethanesulfonate to address elevated ammonia.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
Quickly(?), here's a few reasons why the patent doesn't persuade me that ClorAm-X etc would protect from ammonia in a hobby saltwater setting. (It may, but what's presented isn't convincing at all.)
Looking at the example experiments in the patent:
Example 1 is a freshwater test using 10 Normal NaOH to push the pH of the ammonia chloride solution so high (not measured) that presumably ~100% of the ammonia is NH3, so it can all be measured with the ion selective electrode. (The electrode doesn’t measure total ammonia, NH3+NH4, it measures NH3 only).
The amount of NH3 is 0.5 to 5ppm free ammonia and the pH is who-knows-how high. Reef hobbyists want to handle stuff more in the ballpark of ~0.1ppm NH3 at pH 8.
But even at very high NH3 and very high pH, the product only reduced 0.5ppm NH3 by 10%. The % reductions got better at very high NH3, and worse at the "low" concentrations, suggesting that in our ballpark of ~0.1ppm NH3 and below, the removal might be unobservable in a real saltwater system.
Examples 2, 3, and 4 are test tube reactions directly between high concentrations of ammonium hydroxide and the product. Would the same reactions occur at single digit ppm concentrations in saltwater?
Example 5 is about the dechlorinating property, which it verifiably does.
Example 6 is observation that it doesn't make any weird reactions by adding the product to fresh or saltwater.
No Example 7
Example 8 is that the product itself is not toxic to freshwater fish in high doses.
Example 9 shows that it protects freshwater fish from chlorinated tap water.
Example 10 shows that overdoses of the product don't hurt condy anemones in saltwater.
Example 11 was a freshwater test involving a large number of fish. It seems that the only toxic condition that the fish were subjected to was water changes with tap water, and if I read it right, a normal dechlorinator and ClorAm-x both kept the fish alive (with statistically insignificant exceptions).
Example 12 is saltwater data at some aquarium-relevant ammonia and pH levels that looks like it shows reduction of ammonia. So let’s take a minute and unpack, since it's the most relevant.
First, in one part the experimenter is trying to measure tiny amounts of free ammonia (total ammonia = 1.0, pH 6.0, so free ammonia is 0.00032 ppm NH3 ). Ion-Selective Electrodes like this from Hach (or orion) don’t go near that low - only to 0.01mg/L NH3. To get around the fact that the starting concentration is 1/20th of the normal lower range of these meter and what’s wanted is to measure reduction below that…. here’s what was done: mix the concentration to 1.00ppm TOTAL ammonia, recalibrate the Ion Selective Electrode to tell the meter that it is actually 1.00ppm FREE ammonia (NH3) and then track the reduction with the meter from that point on. This is reported as relative decrease in ammonia. So table 5 is saying that the electrode measured a 32% decrease in free ammonia NH3 from 0.00032 mg/L to 0.00022mg/L. Seems that detection of a decrease of 1 tenth of a part per billion NH3 would be really hard to replicate.
I don’t trust that just calibrating the meter at way below its lower detection limit gets around the lower limits of such devices, but that’s what was done.
Let's just assume for now that the calibration method worked and the meter gave real values, maybe it did. The more fundamental issue is that no mention is made of how pH of saltwater was set to 6.0, 7.0, 8.0, and 9.0 for the various tests, and no mention is made of any attempt to control or measure pH during the application of the product.
This is fundamental because when I measure pH of adding ClorAm-X to saltwater, it drops the pH by a tenth at low doses and several tenths at high doses (with or without ammonia present). @Dan_P also measured a similar pH drop. So did this paper, that needed to buffer the constant low pH while using ClorAm-X in intensive culture.
“ClorAm-X is an alkali metal formaldehydebisulfite that binds to unionized ammonia creating an aminomethanesulfonate salt and thereby reducing TAN. This molecule is nontoxic and does not interfere with the nitrogen cycle (Kuhns 1987). The neutralizing of free ammonia releases a hydrogen ion, lowering pH. To buffer the system, 5 g of sodium bicarbonate/d was added to the ClorAm-X solution”
To see why the pH is a thing to be nitpicky about, take the data from the most applicable situation for the reef hobbyist - Table 11 (Ion Selective Electrode is well within its range here).

Saltwater at 1.020 s.g. was set to total ammonia of 5.0ppm and pH of 8.0. The reduction in measured free ammonia (NH3) that was achieved after 2 hrs was 40%. It had basically leveled off by 30 minutes, with almost no change from 30 min to 2 hours.
I took my tank water, diluted to 1.020s.g. and added 5ppm total ammonia, and measured the pH with ClorAm-X addition. It was initially stable at 7.89 pH, and when adding ClorAm-X, it dropped to 7.74 pH by 2 hours with most of the initial drop recorded in the first ~10 minutes. Running these pH values into the calculator, predicts a reduced NH3 from 0.1272 to 0.0907 or ~30% just from pH. Comparing to table 11’s measured 40% reduction, it’s hard to see what it is that ClorAm-X is supposed to have done to the ammonia. It looks like the “reaction” documented in the most relevant data may have just been a pH drop from ClorAm-X and maybe little else.
But even if it were a completely real NH3-binding reaction and pH were controlled, the data says that reduction of NH3 in a saltwater tank is limited and never actually goes low enough to be acceptable (5ppm to 3ppm total ammonia).
Example 13 shows that they took marine livestock, shipped them in bags from the Virgin Islands to Kansas City over 48hrs. The product was added to every bag and the animals survived. No attempt was made to ship some without the product to show a difference and no measurements of any kind (pH, NH3) were done.
Example 14 is the same exercise as example 12, but in hard freshwater instead of saltwater.
Example 15 is much the same as example 12, but used solid product instead of dissolved. Again, 1ppm total ammonia at pH 6.8 is a very tiny free ammonia amount (0.002 mg/L NH3) and is below detection limit for the NH3 Ion Selective Electrodes that I can find from Hach and Orion.
And that’s all the example data in the patent. And I see nothing that persuades me that it reacts to neutralize ammonia in saltwater in any significant degree, nor was any attempt made to demonstrate that saltwater livestock fared better with the product than without.
So big picture - why bother with something that may do nothing but lower pH a tenth or two? especially when you consider that you can dump in a bottle of Fritz Turbo Start and go from 8ppm to zero in under a day, and Biospira in high doses can be nearly as fast - there’s really no reason I can see to rely on hydroxymethanesulfonate to address elevated ammonia.
I was disappointed to see no controls in the in vivo experiments related to ammonia.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
Patents for chemical syntheses are similarly frustrating.
Because they do not give enough detail to reproduce the experiment? In general a patent must enable a skilled person to reproduce it. Mine give that level of detail, though the synthesis methods may change as the product is scaled up.
- Joined
- Sep 21, 2018
- Messages
- 6,783
- Reaction score
- 7,262
I remember trying to reproduce a betalactam synthesis for a patent infringement case. I was instructed to follow the patent exactly, no innovations to make it work. The synthesis failed. For the chemical synthesis patents I dealt with, this seemed par for the course.Because they do not give enough detail to reproduce the experiment? In general a patent must enable a skilled person to reproduce it. Mine give that level of detail, though the synthesis methods may change as the product is scaled up.
When I saw the ClorAmX patent, there was moment of “maybe I am overly cynical about patents”.
- Joined
- Sep 20, 2018
- Messages
- 1,132
- Reaction score
- 1,100
This is a rebuttal of sorts that I found on another forum. It will probably kill a few brain cells.

 forum.aquariumcoop.com
forum.aquariumcoop.com

A Brief History of Prime
A BIG THANK YOU to @Guppysnail and @Odd Duck for this concept, for the editing of this article and for reviewing it for accuracy and errors! A Brief History of Prime® An abbreviated history of dechlorinators and detoxifiers and their evolution Discussions on whether dechlorination and detoxificat...
- Joined
- May 22, 2016
- Messages
- 6,625
- Reaction score
- 10,228
Nice! Thanks for sharing. It's a masterpiece. I'd have found it thoroughly persuasive if I read it a couple of years ago.This is a rebuttal of sorts that I found on another forum. It will probably kill a few brain cells.

A Brief History of Prime
A BIG THANK YOU to @Guppysnail and @Odd Duck for this concept, for the editing of this article and for reviewing it for accuracy and errors! A Brief History of Prime® An abbreviated history of dechlorinators and detoxifiers and their evolution Discussions on whether dechlorination and detoxificat...forum.aquariumcoop.com
At least until I started trying to measure prime etc effects. Then i would've been very confused.
Lol! Love the titleThis is a rebuttal of sorts that I found on another forum. It will probably kill a few brain cells.

A Brief History of Prime
A BIG THANK YOU to @Guppysnail and @Odd Duck for this concept, for the editing of this article and for reviewing it for accuracy and errors! A Brief History of Prime® An abbreviated history of dechlorinators and detoxifiers and their evolution Discussions on whether dechlorination and detoxificat...forum.aquariumcoop.com
- Joined
- Sep 21, 2018
- Messages
- 6,783
- Reaction score
- 7,262
WOW! Thanks. Definitely fun to read especially after seeing that very little effort that Prime does nothing to ammonia concentration, ditto ClorAmX.This is a rebuttal of sorts that I found on another forum. It will probably kill a few brain cells.

A Brief History of Prime
A BIG THANK YOU to @Guppysnail and @Odd Duck for this concept, for the editing of this article and for reviewing it for accuracy and errors! A Brief History of Prime® An abbreviated history of dechlorinators and detoxifiers and their evolution Discussions on whether dechlorination and detoxificat...forum.aquariumcoop.com
I think when you remove the tangential and irrelevant information, there isn’t much left to the article
SO there are 3 possibilities - all of the science in the article is wrong, or something in your methods is wrong - or both are wrong (meaning you could be right). I don't see much information in either one about 'detoxifying' ammonia. Thats the question 'Does Prime Detoxify ammonia'. It's not how do Seachem alerts and etc work or not work. IMHO - it probably takes a very SMALL amount of ammonia decrease to 'detoxify' ammonia in vivo. The color differences in your experiments to my (poor) eyes - are subtle but there. Thus - IMHO - the question remains - is the detoxification part. Your measurements are not sensitive enough to detect an in vivo difference (with free ammonia) again - only IMHO.Nice! Thanks for sharing. It's a masterpiece. I'd have found it thoroughly persuasive if I read it a couple of years ago.
At least until I started trying to measure prime etc effects. Then i would've been very confused.
Perhaps the reason is decrease in pH - in part. The point is - it de-toxifies free ammonia to a degree. Right?I took my tank water, diluted to 1.020s.g. and added 5ppm total ammonia, and measured the pH with ClorAm-X addition. It was initially stable at 7.89 pH, and when adding ClorAm-X, it dropped to 7.74 pH by 2 hours with most of the initial drop recorded in the first ~10 minutes. Running these pH values into the calculator, predicts a reduced NH3 from 0.1272 to 0.0907 or ~30% just from pH. Comparing to table 11’s measured 40% reduction, it’s hard to see what it is that ClorAm-X is supposed to have done to the ammonia. It looks like the “reaction” documented in the most relevant data may have just been a pH drop from ClorAm-X and maybe little else.
But even if it were a completely real NH3-binding reaction and pH were controlled, the data says that reduction of NH3 in a saltwater tank is limited and never actually goes low enough to be acceptable (5ppm to 3ppm total ammonia).
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
IMHO - it probably takes a very SMALL amount of ammonia decrease to 'detoxify' ammonia in vivo.
I’m not sure what that even means. There is a minimum toxic level, and anything above that is toxic and so to detoxify, at least usefully, one needs to bring the level down from wherever it is, to that non toxic level.
If that toxic level is 1 ppm ammonia, and ammonia is at 3 ppm, one needs to bind up 2 ppm to “detoxify” it.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
Seachem specifically says that pH is not lowered by Prime.Perhaps the reason is decrease in pH - in part. The point is - it de-toxifies free ammonia to a degree. Right?
Seachem - Prime
“ It is non-acidic and will not impact pH”
You need to use 'free ammonia' - which is what I was talking about. However, using your example - you're correct. But - free ammonia depends (as you well know) - pH temperature and an individual fish's tolerance. There is quite a variance - from the literature. The problem is the level of detection. Let's say 0.1 ppm was toxic - but the tests measuring the ammonia have a 'margin of error' of 0.2 ppm - There may be no 'drop' in free ammonia - but there may be a detoxifying effect. I'm not sure why you're disagreeing with me. All I said - is that I have seen no experiments that verify that Prime (etc etc) - do not 'detoxify' ammonia temporarily - for whatever reason - whether thats pH, Prayer or some other reasonI’m not sure what that even means. There is a minimum toxic level, and anything above that is toxic and so to detoxify, at least usefully, one needs to bring the level down from wherever it is, to that non toxic level.
If that toxic level is 1 ppm ammonia, and ammonia is at 3 ppm, one needs to bind up 2 ppm to “detoxify” it.
Yet some of the experiments quoted suggested that it did - so those must be incorrect then - thanks. I quoted someone else's post - I did not do the study myselfSeachem specifically says that pH is not lowered by Prime.
Seachem - Prime
www.seachem.com
“ It is non-acidic and will not impact pH”
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
SO there are 3 possibilities - all of the science in the article is wrong, or something in your methods is wrong - or both are wrong (meaning you could be right). I don't see much information in either one about 'detoxifying' ammonia. Thats the question 'Does Prime Detoxify ammonia'. It's not how do Seachem alerts and etc work or not work. IMHO - it probably takes a very SMALL amount of ammonia decrease to 'detoxify' ammonia in vivo. The color differences in your experiments to my (poor) eyes - are subtle but there. Thus - IMHO - the question remains - is the detoxification part. Your measurements are not sensitive enough to detect an in vivo difference (with free ammonia) again - only IMHO.
You are pointing out lots of perceived flaws in testing (I don’t agree with them, but that’s not my point here).
Why do you accept claims from Seachem that are not supported by any data by Seachem or any independent tester? Nothing, except reports from some users that used it and “believed” it to be useful.
That’s an awfully low threshold for acceptance, and would lead one to accept all kinds of wild claims for products from herbal remedies to copper infused socks,
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,983
- Reaction score
- 64,416
You need to use 'free ammonia' - which is what I was talking about. However, using your example - you're correct. But - free ammonia depends (as you well know) - pH temperature and an individual fish's tolerance. There is quite a variance - from the literature. The problem is the level of detection. Let's say 0.1 ppm was toxic - but the tests measuring the ammonia have a 'margin of error' of 0.2 ppm - There may be no 'drop' in free ammonia - but there may be a detoxifying effect. I'm not sure why you're disagreeing with me. All I said - is that I have seen no experiments that verify that Prime (etc etc) - do not 'detoxify' ammonia temporarily - for whatever reason - whether thats pH, Prayer or some other reason
Why assume I wasn’t? Pick any number you want for the toxic level. The product needs to bind everything above that level. Not some tiny amount. Binding all of the 0.1 ppm free ammonia when total ammonia is 2 ppm, will still leave 0.095 ppm free ammonia when it reequilibrates. That’s how the chemistry works.
Now you are claiming it detoxifies without reducing free ammonia? When Seachem specifically says that is how and why it works?
Why scramble so hard to explain away real data when no conflicting data is presented?
Last edited:
I'll simplify it - There is data - that suggests that Prime and other products do not reduce ammonia. I'm not at all convinced about their sensitivity or sensitivity to make those conclusions. Perhaps they are right - perhaps they are incorrect - But - you're kind of dancing around my point - that no in-vivo studies have been done on this forum. There are multiple people (uncontrolled) - who CLAIM to have moribund fish come around quickly after adding Prime et al. Could those be coincidence? Are there others with moribund fish who have added prime with no effect - probably. I don't know. Is Seachem somewhat inconsistent - yes. Has seachem claimed to do in vivo testing yes they have (in a private discussion). The patent literature also suggests that it's possible. So - I'm not sure why you think I'm scrambling. I'm agreeing with your basic premise lol. I said the results here suggest that Prime does not 'remove' free ammonia.Why assume I wasn’t? Pick any number you want for the toxic level. The product needs to bind everything above that level. Not some tiny amount. Binding all of the 0.1 ppm free ammonia when total ammonia is 2 ppm, will still leave 0.095 ppm free ammonia when it reequilibrates. That’s how the chemistry works.
Now you are claiming it detoxifies without reducing free ammonia? When Seachem specifically says that is how and why it works?
Why scramble so hard to explain away real data when no conflicting data is presented?
Similar threads
- Replies
- 2
- Views
- 123
- Replies
- 12
- Views
- 142
- Replies
- 29
- Views
- 425
- Replies
- 38
- Views
- 917

















