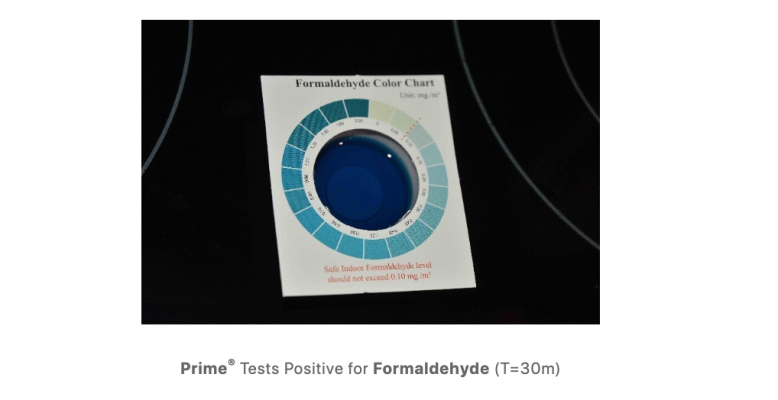
I found this prior video posted of the tank from December

 youtube.com
youtube.com
Prime didn’t have anything to resolve if that’s the system the non digital reading came from

Jawfish full recovery?
Prime didn’t have anything to resolve if that’s the system the non digital reading came from
Last edited: