The alternative is that Prime does nothing to neutralise, bind, reduce, obliterate or magic away ammonia...
It's already been proven without any doubt that this is the case.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
The alternative is that Prime does nothing to neutralise, bind, reduce, obliterate or magic away ammonia...
Prime says it "detoxifies" ammonia. I can categorically say that I have no idea how one would do that or even have enough knowledge to guess. But the only thing I see in this thread is proof that if Prime does anything at all re: ammonia it isn't showing up on a test kit and probably doesn't "bind" ammonia.
I can also say that I have seen many fish showing classic signs of ammonia toxicity in tanks I know are not cycled properly perk up almost instantly when Prime is added. Perhaps, some form of fish Xanax and opium is in Prime so they don't care whether they live or die and they can't feel ammonia burning through their gills.
"Proof" is an annoying word that means too many different things to people.It's already been proven
I propose folks mean “incontrovertible proof” when they say “proof”."Proof" is an annoying word that means too many different things to people.
So let's just state the issue in terms of evidence, and what can and can't be verified...
- Prime (and many other ammonia "treatments") are dechlorinators, and thus interfere with the fundamental mechanism of Total Ammonia chemical tests - so they cannot be verified by the common API, Red Sea, Hanna, Hach etc kits.
- They claim to bind ammonia which would change the ammonia in a way that it would not pass through a gas permeable membrane in the ammonia-sensing films. But Seachem ammonia alert badges, multi-test disks, and seneye films all show ammonia unchanged. This indicates NH3 amount is unchanged and still present in gas form as it was before. So it cannot be verified by that mechanism either.
- No evidence is provided by seachem as to the chemical or how it might react with ammonia, so it cannot be verified by published research on such chemical interactions.
- No evidence is provided that Prime protects organisms subjected to lethal ammonia statistically moreso than not treating them at all, so it cannot be verified by that mechanism.
- I subjected aquarium amphipods to lethal ammonia and they were incapacitated and died at the same rate with an amount of Prime proportional to the ammonia, as with no protection at all - so Prime could not be verified by that mechanism either.
Contrast that with the fact that there are multiple things you could do or add that would lower toxic free ammonia and be absolutely verifiable by some or all of the above means.
Water changes,
lower PH,
Some fast Nitrifier products (Fritz turbostart),
Carbon Dose to grow bacteria,
Lighted Algae.
If there were an actual toxic ammonia emergency, Prime makes no sense in light of the above.

How does everyone feel about ion chromatography in this situation? There's a lab near me and I should be able to easily create some samples.
Surely if Prime is binding ammonia it'll be detectable via ion chromatography.
Maybe it works by freeing you from the fear of ammonia, and it's actually the fear of ammonia that harms livestock.
It would seem that somebody who was very interested in demonstrating that it worked (such as Seachem) could use ion chromatography to show the decrease or disappearance of the ammonia peak after treatment by Prime.
https://appslab.thermofisher.com/App/3325/inorganic-cations-ammonium-waters-using-ic
As suggested earlier...
Seems very straightforward and cheap considering the value of the demonstration to Seachem.
...interestingly, we now have multiple ICP vendors who are running ion chromatography on our saltwater samples anyway for determination of nitrate (Oceamo also uses it for Sulfate and some other ions). Ammonia is not an in-demand measurement for hobbyists, but I wonder if the ammonia peak would be present on those same ICP runs.
Bad decisions are made under stress. Adding a placebo (Prime) and doing nothing else might explain the success of Prime.Maybe it works by freeing you from the fear of ammonia, and it's actually the fear of ammonia that harms livestock.
I still think we are chasing Big Foot when we are holding out hope someone has convincing data.Be nice if someone somewhere claiming it worked showed a single bit of experimental evidence it worked, or a chemical rationale of how it might possibly work.
Right. My joke is only half a joke. "Placebo and let it ride" probably fixes a lot of problems encountered in the Hobby and may overall have better outcomes than poor decisions hobbyists make when trying to aggressively "fix" a problem.Bad decisions are made under stress. Adding a placebo (Prime) and doing nothing else might explain the success of Prime.
Same thoughts here.I'm sure I posted this before but can't find it. Anyway, it's a review of all the ammonia speciation methods including IC.
I wonder if it could be as simple as placing a beaker of ammonia solution and a beaker of water in an airtight compartment. Gaseous ammonia should end up in the beaker of water. Prime would have to render the ammonia non-volatile to prevent diffusion through gills.

Maybe it works by freeing you from the fear of ammonia, and it's actually the fear of ammonia that harms livestock.
It would seem that somebody who was very interested in demonstrating that it worked (such as Seachem) could use ion chromatography to show the decrease or disappearance of the ammonia peak after treatment by Prime.
https://appslab.thermofisher.com/App/3325/inorganic-cations-ammonium-waters-using-ic
As suggested earlier...
Seems very straightforward and cheap considering the value of the demonstration to Seachem.
...interestingly, we now have multiple ICP vendors who are running ion chromatography on our saltwater samples anyway for determination of nitrate (Oceamo also uses it for Sulfate and some other ions). Ammonia is not an in-demand measurement for hobbyists, but I wonder if the ammonia peak would be present on those same ICP runs.
| IC Eluent Concentration | 30 mM Methanesulfonic acid |
|---|
Here's proof of concept. I really like the simplicity of this demonstration to explain what we mean when we say the NH3 is still there and it's still a gas.I wonder if it could be as simple as placing a beaker of ammonia solution and a beaker of water in an airtight compartment. Gaseous ammonia should end up in the beaker of water. Prime would have to render the ammonia non-volatile to prevent diffusion through gills.

Here's proof of concept. I really like the simplicity of this demonstration to explain what we mean when we say the NH3 is still there and it's still a gas.
I took tank water and added ammonia drops to ~4ppm.
One part was untreated, one part I added 4x dose Prime and mixed. Then I poured the mixed 40mL into 50mL tubes, covered the tops with a paper towel, put a couple of seachem NH3 films on the paper towel and covered the whole thing in parafilm. The water never touched the paper towel, the NH3 films are simply responding to the gases over the water.
Top pic is after 1-2 hours. Bottom pic is overnight.
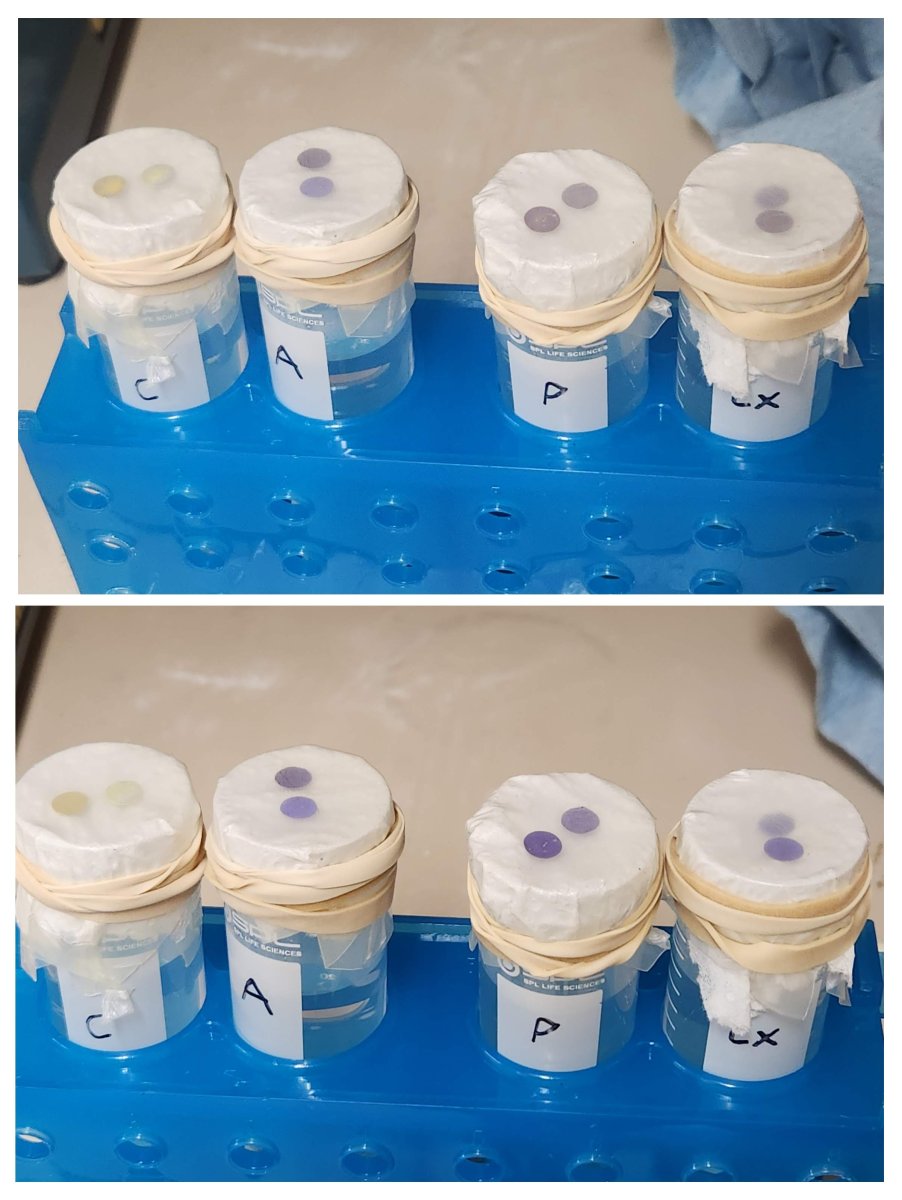
Left to right Tubes: Control - no ammonia, Ammonia untreated, Ammonia + 4x Prime, (and as bonus the rightmost is with ClorAm-X)
I did this because it was faster than waiting for NH3 to diffuse out of one sample of water and into another. But this strongly suggests that's exactly what would happen, whether you do or don't treat with Prime (or likely other products).
Right. I didn't know how much response (if any) there would be. I didn't expect 4ppm in the water to literally max out the color on the films in the air above it. Those tiny dots are good at detection but annoying for quantification.This is a very good experiment, but it is lacking in a dose dependent control. For example, if the Prime or Chlor-Am-X reduced the free ammonia by 90%, would the response of the badge be the same?

Just how much of this gas can be reduced, by vigorous aeration, say a massive air stone?I really like the concept of the air experiment to demonstrate how much the free ammonia has been reduced.
Adding a second container of water into it makes the geometry and even convection currents inside the overall container due to small temperature differences of great importance, unless you run the experiment for times long enough to know you are at equilibrium.

Just how much of this gas can be reduced, by vigorous aeration, say a massive air stone?
Edit - presumably, as the NH3 is gassed of it’s replaced by a slightly lower concentration due the pH relationship of NH4/NH3, so all of it?
a couple of simple-ish physical things I've checked to see if it removes total ammonia...Just how much of this gas can be reduced, by vigorous aeration, say a massive air stone?
Edit - presumably, as the NH3 is gassed of it’s replaced by a slightly lower concentration due the pH relationship of NH4/NH3, so all of it?
