So from yesterday midday until this evening I’ve had a decline in pH. My scrubber media is only half depleted. I really couldn’t figure out why pH was lower than normal.
Well, yesterday my wife got her latest shipment of Nutrisystem frozen meals. It comes in a large styrofoam cooler with a giant brick of dry ice. I was supposed to take the dry ice to school with me so I can make dry ice bubbles with my students. I forgot. When I got home my wife was complaining of a headache and told me to get the cooler out. That’s when I realized I forgot the dry ice. When I popped the top on the cooler, the dry ice had completely sublimated. @Randy Holmes-Farley can a large brick of dry ice have such an effect on the tanks pH? Usually we put the cooler and dry ice in the garage or I leave the dry ice on the driveway. This is the first time we let it sit in the house all day.
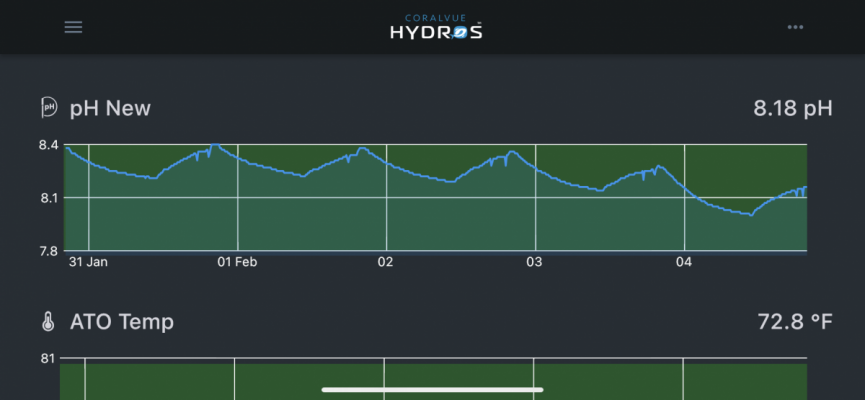
Well, yesterday my wife got her latest shipment of Nutrisystem frozen meals. It comes in a large styrofoam cooler with a giant brick of dry ice. I was supposed to take the dry ice to school with me so I can make dry ice bubbles with my students. I forgot. When I got home my wife was complaining of a headache and told me to get the cooler out. That’s when I realized I forgot the dry ice. When I popped the top on the cooler, the dry ice had completely sublimated. @Randy Holmes-Farley can a large brick of dry ice have such an effect on the tanks pH? Usually we put the cooler and dry ice in the garage or I leave the dry ice on the driveway. This is the first time we let it sit in the house all day.















