I watched this video as it was something that interested me. I run a full saturated calcium reactor on my 220 gallon sps tank. My ph at night can be as low as 7.65 and a high in the day of 7.95 ( all ph probes calibrated and correct). For someone in my situation what would people suggest for raising the ph? I seriously looked into dosing potassium hydroxide if it was going to help. Maybe Randy and other people can help me out here. ThanksOK, KOH and Ca(OH)2.
There is nothing special or particularly useful about potassium hydroxide. There are very few situations where I'd use it. Maybe none.
Hydroxide in any form (sodium, potassium, calcium) is a way to boost alkalinity and also pH. It has about twice the pH raising effect per unit of alk added as does sodium carbonate.
No form of hydroxide can raise pH without raising alkalinity.
If the goal is to make an alk corrective boost, then one could use bicarbonate (with very slight pH lowering), carbonate (with substantial pH raising), hydroxide (with bigger pH raising) or any mixture of them that you want (though mixing hydroxide and bicarbonate just gives carbonate).
For routine use, one of course also wants to add calcium. That can be done using any of these along with calcium chloride in a DIY or commercial two aprt or Balling, or one "one part" systems that combine calcium and alkalinity into a single entity such as calcium hydroxide, calcium acetate, or calcium formate.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Potassium Hydroxide Dosing??
- Thread starter special-ops-s2k
- Start date
- Tagged users None
- Joined
- Apr 28, 2019
- Messages
- 433
- Reaction score
- 753
Do you dose Kalkwasser at all? I'd probably recommend you start with that, it's REALLY helped me boost and stabilize my PH... Then I'd guess that Randy will suggest Sodium Hydroxide over Potassium hydroxide if you need a larger boost than what Kalk can provide... Based on his previous comments and suggestions, that was my takeaway.
Let us know what you decide to go with!
Let us know what you decide to go with!
I watched this video as it was something that interested me. I run a full saturated calcium reactor on my 220 gallon sps tank. My ph at night can be as low as 7.65 and a high in the day of 7.95 ( all ph probes calibrated and correct). For someone in my situation what would people suggest for raising the ph? I seriously looked into dosing potassium hydroxide if it was going to help. Maybe Randy and other people can help me out here. Thanks
Thanks for your reply, I live in the uk and haven’t had any experience with kalkwasser as I don’t believe it’s something that is used massively over here. I don’t have a kalkstirrer or peristaltic pump.Do you dose Kalkwasser at all? I'd probably recommend you start with that, it's REALLY helped me boost and stabilize my PH... Then I'd guess that Randy will suggest Sodium Hydroxide over Potassium hydroxide if you need a larger boost than what Kalk can provide... Based on his previous comments and suggestions, that was my takeaway.
Let us know what you decide to go with!
- Joined
- Apr 28, 2019
- Messages
- 433
- Reaction score
- 753
Thanks for your reply, I live in the uk and haven’t had any experience with kalkwasser as I don’t believe it’s something that is used massively over here. I don’t have a kalkstirrer or peristaltic pump.
Yeah I'm currently using a Kalk stirrer, fed with a Kamoer FX-STP pump... I use BRS branded Kalk powder and it really does boost the ph significantly. My PH journey started with lows of 7.85ish at night and highs never touching 8.0 in the days... I plumbed in a Co2 scrubber and that got me 8.07 at night and 8.21 in the daytime peak! I wasn't satisfied with that however and added the Kalkwasser Stirrer, now I have it dialed in at 8.4 at the low point and 8.49 at the daily peak! The chart is basically flat and the corals are sucking up the Alkalinity, you can DEFINITELY see a large difference in consumption when the Ph is basically 8.4 24/7
I actually hit 8.51-8.52 in my early experiment phase dialing it in! So I now have the apex cut power to the feed pump if the ph hits 8.49 and start it back up if it drops to 8.47, this basically pegs my ph at 8.48-8.49 most of the photo period...
Good luck!
- Joined
- Jan 29, 2020
- Messages
- 184
- Reaction score
- 137
Kalk is great! Its not enough to keep my PH where id like it but Kalk alone keeps me at 450 Ca and 9.5 DKH with one of the BRS 2 ml dosers
I watched this video as it was something that interested me. I run a full saturated calcium reactor on my 220 gallon sps tank. My ph at night can be as low as 7.65 and a high in the day of 7.95 ( all ph probes calibrated and correct). For someone in my situation what would people suggest for raising the ph? I seriously looked into dosing potassium hydroxide if it was going to help. Maybe Randy and other people can help me out here. Thanks
Kalk stir and a slow dosing pump will definitely worth the investment. It not only add high pH solution, but also added alk and ca that reduce the demand from calcium reactor, thus reduce the among of low pH solution going in.
But a quick change is to put the output of calcium reactor directly into the skimmer, so that it's gassed out as much as possible there. The way Chris suggest is to drill a hole on the skimmer cup and run the line directly into the skimmer body. That alone brought him a 0.1 increase in pH.
Yeah I get that. I just didn’t want to spend more money on a kalk stirer and versa pump or something similar after I just spent a load of money on the carxKalk stir and a slow dosing pump will definitely worth the investment. It not only add high pH solution, but also added alk and ca that reduce the demand from calcium reactor, thus reduce the among of low pH solution going in.
But a quick change is to put the output of calcium reactor directly into the skimmer, so that it's gassed out as much as possible there. The way Chris suggest is to drill a hole on the skimmer cup and run the line directly into the skimmer body. That alone brought him a 0.1 increase in pH.
It is so sad (IMO) that this is getting such publicity.
There is zero reason to use potassium hydroxide.
Food and other high grades of sodium hydroxide are better choices for a high pH alkalinity supplement, without concerns about excessive potassium.
If you need potassium, using cheap food grade potassium chloride will allow far better control than to try to tie alkalinity dosing and potassium supplementing together.
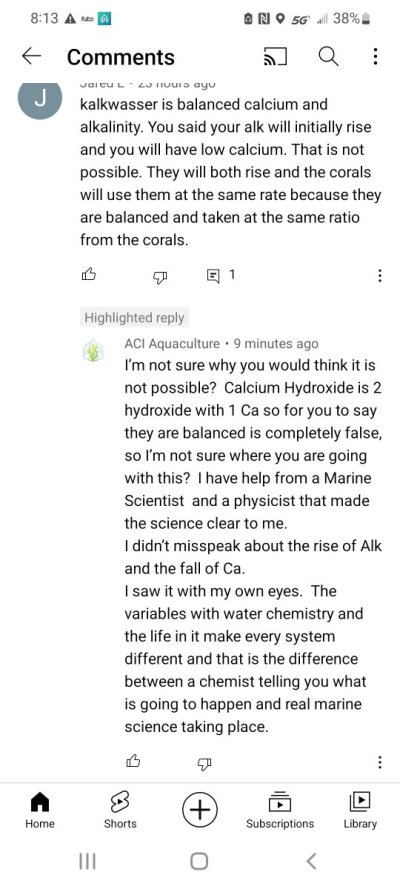
I have been listening to this guy from aci aquaculture and he is getting some major and very basic concepts wrong. He has a video on how to dose kalkwasser to raise ph. He says match your tanks evaporation, whatever that is. And dose kalk based on ph alone. He says your alkalinity will rise sometimes up to 15 dkh. Then says your calcium will go down so you may need to dose calcium. So you're adding enough kalk to raise your alk to 15 dkh and he says your calcium will be low!!
Not to mention he says this works with all tanks. You could have no corals or a 2 dkh a day consumption, so how this would work for both is beyond me. If you have a high evaporation rate, low ph, and no alk consumption, you would be pouring in a lot of kalk to boost ph to 8.3. Seems like a recipe for complete disaster.
I mean what am I missing here. Either I am wrong or a youtube influencer with a large aquaculture business is wrong.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
I have been listening to this guy from aci aquaculture and he is getting some major and very basic concepts wrong. He has a video on how to dose kalkwasser to raise ph. He says match your tanks evaporation, whatever that is. And dose kalk based on ph alone. He says your alkalinity will rise sometimes up to 15 dkh. Then says your calcium will go down so you may need to dose calcium. So you're adding enough kalk to raise your alk to 15 dkh and he says your calcium will be low!!
Not to mention he says this works with all tanks. You could have no corals or a 2 dkh a day consumption, so how this would work for both is beyond me. If you have a high evaporation rate, low ph, and no alk consumption, you would be pouring in a lot of kalk to boost ph to 8.3. Seems like a recipe for complete disaster.
I mean what am I missing here. Either I am wrong or a youtube influencer with a large aquaculture business is wrong.
Odd that anyone would claim calcium would decline. I don’t see how it can by dosing calcium hydroxide. No matter how high you drive alk or how much precipitation of calcium carbonate you get, calcium cannot decline if alk is equal or higher than where you started.
Much more likely is that calcium climbs substantially in both the short term (matching the alk rise) and long term (from magnesium and strontium getting into deposited calcium carbonate in place of some of the calcium).
So this guy from aci aquaculture actually just responded to me on this exact topic. See attached.Odd that anyone would claim calcium would decline. I don’t see how it can by dosing calcium hydroxide. No matter how high you drive alk or how much precipitation of calcium carbonate you get, calcium cannot decline if alk is equal or higher than where you started.
Much more likely is that calcium climbs substantially in both the short term (matching the alk rise) and long term (from magnesium and strontium getting into deposited calcium carbonate in place of some of the calcium).
Attachments
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
So this guy from aci aquaculture actually just responded to me on this exact topic. See attached.
He does not understand simple chemistry. I cannot see the whole response, but the reason that two hydroxides balances one calcium is because calcium carbonate consists of one mole of calcium and two moles of alkalinity.
This is a great example of internet misinformation.He does not understand simple chemistry. I cannot see the whole response, but the reason that two hydroxides balances one calcium is because calcium carbonate consists of one mole of calcium and two moles of alkalinity.
Someone reads something in which they don't understand the basic underlying science, believes it, and then thinks they're an expert on the subject. Then they start making posts, videos, etc. reinforcing and spreading the misinformation to others.
Luckily, we have subject experts to clear up most of these misconceptions but unfortunately, many people don't stop to check the credentials of the source of their information.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
I can see the whole response now on my PC. I take it I'm the chemist he says doesn't understand what is going on, but his marine scientist and physicist friends understand the chemistry better. I have nothing against either one (my dad is a physicist), but this is a chemistry question for which the answer is exactly known, backed up by decades of seeing what does happen in my tank (I used kalkwasser only for 20 years) and hundreds, maybe thousands of reefers using kalkwasser who I have discussed the issue with over those same decades in thousands of online threads.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
I sent them an email this morning, I'll let folks know if there is any response:
Hello ACI,
You may or may not care, but your reputation is suffering by putting out misinformation about the effects of kalkwasser (calcium hydroxide solution) dosing. I am likely the chemist that you claim doesn’t understand the chemistry of your claims, but I think you need to read up on the effects of kalkwasser on calcium and alkalinity long term. This misinformation is now the topic of a public thread in my forum at Reef2Reef.
Kalkwasser is close to balanced for calcium and alkalinity dosing, and actually delivers a slight EXCESS of calcium over time, not a deficiency, when the alk and calcium are used to make coral skeletons and other calcium carbonate materials in a reef tank. Anything different that you observed was for other reasons. These other reasons can include alkalinity additions from some other means, such as declining nitrate or water changes with a high alk mix.
Why is kalkwasser balanced?
Calcium hydroxide delivers one unit of calcium and two units of alkalinity (2 OH-). Calcium carbonate contains one unit of calcium and two units of alkalinity (one carbonate = two units of alkalinity, which comes from TWO hydroxide ions):
Ca(OH)2 --> Ca++ + 2OH-
OH– + CO2 → HCO3–
OH– + HCO3– → CO3— + H2O
Ca++ + CO3-- --> CaCO3
Why is it not perfectly balanced for use in a reef aquarium long term? Because a small amount of magnesium and strontium get into the calcium carbonate in place of some of the calcium. Thus, it is a little less than one calcium per two units of alkalinity to make coral skeletons, and if you use kalkwasser to deliver all alkalinity needed by the tank, and there are not other things impacting the values such as water changes, then calcium will rise long term. This has been demonstrated thousands of times, including in my own tank where I used only kalkwasser for 20 years.
Thanks for listening and Happy reefing!
Randy Holmes-Farley
PS. This was your comment containing the misinformation:
Hello ACI,
You may or may not care, but your reputation is suffering by putting out misinformation about the effects of kalkwasser (calcium hydroxide solution) dosing. I am likely the chemist that you claim doesn’t understand the chemistry of your claims, but I think you need to read up on the effects of kalkwasser on calcium and alkalinity long term. This misinformation is now the topic of a public thread in my forum at Reef2Reef.
Kalkwasser is close to balanced for calcium and alkalinity dosing, and actually delivers a slight EXCESS of calcium over time, not a deficiency, when the alk and calcium are used to make coral skeletons and other calcium carbonate materials in a reef tank. Anything different that you observed was for other reasons. These other reasons can include alkalinity additions from some other means, such as declining nitrate or water changes with a high alk mix.
Why is kalkwasser balanced?
Calcium hydroxide delivers one unit of calcium and two units of alkalinity (2 OH-). Calcium carbonate contains one unit of calcium and two units of alkalinity (one carbonate = two units of alkalinity, which comes from TWO hydroxide ions):
Ca(OH)2 --> Ca++ + 2OH-
OH– + CO2 → HCO3–
OH– + HCO3– → CO3— + H2O
Ca++ + CO3-- --> CaCO3
Why is it not perfectly balanced for use in a reef aquarium long term? Because a small amount of magnesium and strontium get into the calcium carbonate in place of some of the calcium. Thus, it is a little less than one calcium per two units of alkalinity to make coral skeletons, and if you use kalkwasser to deliver all alkalinity needed by the tank, and there are not other things impacting the values such as water changes, then calcium will rise long term. This has been demonstrated thousands of times, including in my own tank where I used only kalkwasser for 20 years.
Thanks for listening and Happy reefing!
Randy Holmes-Farley
PS. This was your comment containing the misinformation:
Were talking 2 different animals here. How many gallons of water is Chis maintaining & how many corals is Chris growing and taking care of. And how many gallons and corals is Randy maintaining? I don't belive for 1 minute that Chris is trying to mislead anyone,hes only trying to help.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
Were talking 2 different animals here. How many gallons of water is Chis maintaining & how many corals is Chris growing and taking care of. And how many gallons and corals is Randy maintaining? I don't belive for 1 minute that Chris is trying to mislead anyone,hes only trying to help.
Nonsense. I don’t know what he is trying to do, but he is spouting misinformation that is detrimental to anyone who believes and acts on false information.
Do you not care that what he says is simply wrong?
it’s not a difference of opinion or scale. It’s truth vs untruth. That should matter, IMO.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
Were talking 2 different animals here. How many gallons of water is Chis maintaining & how many corals is Chris growing and taking care of. And how many gallons and corals is Randy maintaining? I don't belive for 1 minute that Chris is trying to mislead anyone,hes only trying to help.
Do you believe that in any system anywhere, that kalkwasser is unbalanced to deliver too little calcium because it adds two hydroxide ions for one calcium?
No one's questioning Chris's ability to keep Coral. That's not the issue here.Were talking 2 different animals here. How many gallons of water is Chis maintaining & how many corals is Chris growing and taking care of. And how many gallons and corals is Randy maintaining? I don't belive for 1 minute that Chris is trying to mislead anyone,hes only trying to help.
In fact, your post is perfect evidence of how people will believe other people on the internet based on non-relevant experience and expertise. Just because somebody is good at something, doesn't make them an expert in all related material and areas. Here he is simply spreading misinformation in an area that is outside of his expertise.
Awesome! Interesting development occurred. I commented on his reply on youtube before you responded on reef2reef. I basically said exactly what you said. Then I referenced an old thread you commented on that backed up my claim. They have deleted that comment. I made no comments that could have been considered anything but respectful. Basically just proved he was wrong. I was literally silenced because I made him look bad. The youtube channel that silenced me is reefs and I will boycott this channel from now on and recommend everybody else do the same.I sent them an email this morning, I'll let folks know if there is any response:
Hello ACI,
You may or may not care, but your reputation is suffering by putting out misinformation about the effects of kalkwasser (calcium hydroxide solution) dosing. I am likely the chemist that you claim doesn’t understand the chemistry of your claims, but I think you need to read up on the effects of kalkwasser on calcium and alkalinity long term. This misinformation is now the topic of a public thread in my forum at Reef2Reef.
Kalkwasser is close to balanced for calcium and alkalinity dosing, and actually delivers a slight EXCESS of calcium over time, not a deficiency, when the alk and calcium are used to make coral skeletons and other calcium carbonate materials in a reef tank. Anything different that you observed was for other reasons. These other reasons can include alkalinity additions from some other means, such as declining nitrate or water changes with a high alk mix.
Why is kalkwasser balanced?
Calcium hydroxide delivers one unit of calcium and two units of alkalinity (2 OH-). Calcium carbonate contains one unit of calcium and two units of alkalinity (one carbonate = two units of alkalinity, which comes from TWO hydroxide ions):
Ca(OH)2 --> Ca++ + 2OH-
OH– + CO2 → HCO3–
OH– + HCO3– → CO3— + H2O
Ca++ + CO3-- --> CaCO3
Why is it not perfectly balanced for use in a reef aquarium long term? Because a small amount of magnesium and strontium get into the calcium carbonate in place of some of the calcium. Thus, it is a little less than one calcium per two units of alkalinity to make coral skeletons, and if you use kalkwasser to deliver all alkalinity needed by the tank, and there are not other things impacting the values such as water changes, then calcium will rise long term. This has been demonstrated thousands of times, including in my own tank where I used only kalkwasser for 20 years.
Thanks for listening and Happy reefing!
Randy Holmes-Farley
PS. This was your comment containing the misinformation:

Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,814
- Reaction score
- 64,235
Awesome! Interesting development occurred. I commented on his reply on youtube before you responded on reef2reef. I basically said exactly what you said. Then I referenced an old thread you commented on that backed up my claim. They have deleted that comment. I made no comments that could have been considered anything but respectful. Basically just proved he was wrong. I was literally silenced because I made him look bad. The youtube channel that silenced me is reefs and I will boycott this channel from now on and recommend everybody else do the same.
You rabble rouser. How dare you challenge them. lol
IMO, if a statement cannot withstand polite challenges, it is probably a faulty statement.
Folks are always welcome to post contrary opinions and ideas and such here. If I think there’s a problem with what was posted, I’ll challenge it back and explain why, but nothing is ever removed just because it disagrees with someone else (only terms of service violations lead to post removal).
Similar threads
- Replies
- 4
- Views
- 150
- Replies
- 4
- Views
- 185
- Replies
- 7
- Views
- 346
- Replies
- 3
- Views
- 209