Need some advice. Tank is two years old running well. I have an auto doser that doses BRS soda ash and BRS calcium chloride. For months this kept them stable
at 9 Alk and 450 calcium. My magnesium has always tested high at 1520 or 1480 since I started the tank. Now over the last weeks this has changed. Every week I now test 7.8 alk. Everytime I dose back up to 9. I increased the dosing from to 40 ml daily to 50 ml daily last week and again today it tested at 7.8. I Dosed it back up to 9 and now I upped the dosing to 70 ml and upped the dosing for calcium from 30 ml daily to 42 ml daily. I have checked the doser and it is calibrated and doses at the right times. 5 times a day for alk and 6 times a day for calcium. I assume this change in alk and calcium has something to do with the drop in magnesium. Should I start dosing magnesium? What is the best product to use for this?
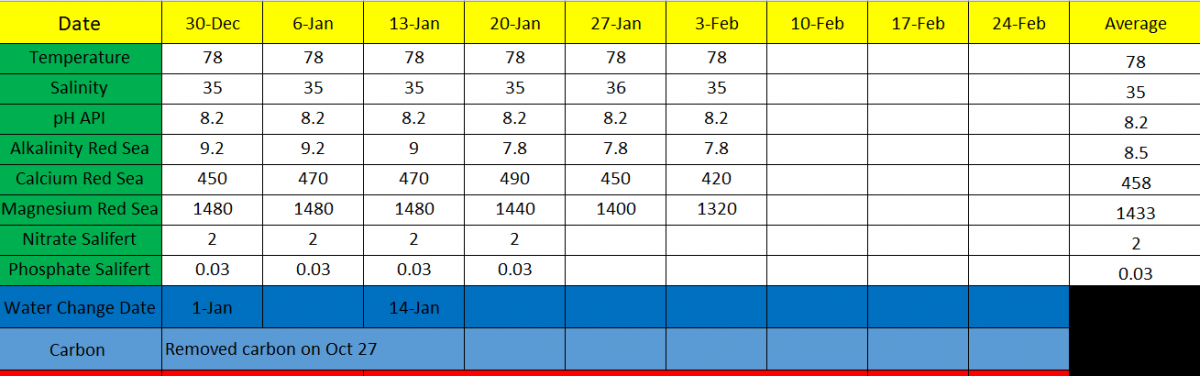
This is my spread sheet for keeping track of my test results and I have four months worth of data that I can post if that will help. I have no idea why the rise in calcium for Jan 6 through Jan 20 but after that it started steadily dropping.
at 9 Alk and 450 calcium. My magnesium has always tested high at 1520 or 1480 since I started the tank. Now over the last weeks this has changed. Every week I now test 7.8 alk. Everytime I dose back up to 9. I increased the dosing from to 40 ml daily to 50 ml daily last week and again today it tested at 7.8. I Dosed it back up to 9 and now I upped the dosing to 70 ml and upped the dosing for calcium from 30 ml daily to 42 ml daily. I have checked the doser and it is calibrated and doses at the right times. 5 times a day for alk and 6 times a day for calcium. I assume this change in alk and calcium has something to do with the drop in magnesium. Should I start dosing magnesium? What is the best product to use for this?
This is my spread sheet for keeping track of my test results and I have four months worth of data that I can post if that will help. I have no idea why the rise in calcium for Jan 6 through Jan 20 but after that it started steadily dropping.



















