Why would you question a standard practice that is used not only in the hobby but in most places where probes are used? When evaluating pH in a reef tank, the difference of .2 at the low end of the range can make a big difference. A two-point calibration reduces the risk of the reading being incorrect.If your buffer 7.0 is off by 0.5 and you do a 2 point calibration - this would not save your day.. In my example the 7.1 solution would 7.6 with single point calibration and 7.58 with 2 point calibration - how is that helping you?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Tested pH with lab pH meter and it's 7.5!
- Thread starter KonradTO
- Start date
- Tagged users None
You probably don't have a mathematical mindset to be on the same wave with me.. You initially questioned the reading of this post by suggesting to do the 2 point calibration. I (mathematically) argued that in this particular instance the error of measurement is not significant and whether or not such 2 point calibration was done is pretty much irrelevant: PH 7.5 being the range of [7.45-7.55] is all you would get and irrespective - it's a low PH reading that needs to be dealt with..Why would you question a standard practice that is used not only in the hobby but in most places where probes are used? When evaluating pH in a reef tank, the difference of .2 at the low end of the range can make a big difference. A two-point calibration reduces the risk of the reading being incorrect.
Let me ask you a counter-question: do you calibrate your probe every time you take PH measurement (i.e. every second)? It's the best practive to calibrate your probe to ensure it's most accurate..
Let me pre-empt your answer: why don't you?
Last edited:
Moving on... I would suggest the OP do some research on how much CO2 it would take to drop 35 ppt (~1.025 SG) seawater, @ say 80 degrees, with an alkalinity in the 7.5 dKh range to a pH of 7.5. I'll suggest that the amount exceeds that found outdoors or even in a reasonable ventilated but closed room. For that reason, I suggest that something else might be wrong like:You probably don't have a mathematical mindset to be on the same wave with me.. You initially questioned the reading of this post by suggesting to do the 2 point calibration. I (mathematically) argued that in this particular instance the error of measurement is not significant and whether or not such 2 point calibration was done is pretty much irrelevant: PH 7.5 being the range of [7.45-7.55] is all you would get and irrespective - it's a low PH reading that needs to be dealt with..
Let me ask you a counter-question: do you calibrate your probe every time you take PH measurement (i.e. every second)? It's the best practive to calibrate your probe to ensure it's most accurate..
Let me pre-empt your answer: why don't you?
1. The probe needs to be properly calibrated;
2. The salinity or alkalinity is not as high as thought;
3. The dissolved organic load is extremely high; or
3. There is some acidic compound being introduced to the tank.
Good plan!Moving on... I would suggest the OP do some research on how much CO2 it would take to drop 35 ppt (~1.025 SG) seawater, @ say 80 degrees, with an alkalinity in the 7.5 dKh range to a pH of 7.5. I'll suggest that the amount exceeds that found outdoors or even in a reasonable ventilated but closed room. For that reason, I suggest that something else might be wrong like:
1. The probe needs to be properly calibrated;
2. The salinity or alkalinity is not as high as thought;
3. The dissolved organic load is extremely high; or
3. There is some acidic compound being introduced to the tank.
Forgot to add... IME, gas exchange is not usually an issue in reasonably operating systems. Even in those with tops. For that reason, I wouldn't include it in the top 5 potential causes of the depressed pH level.
Without 2 point calibration the slope can be way off.You probably don't have a mathematical mindset to be on the same wave with me.. You initially questioned the reading of this post by suggesting to do the 2 point calibration. I (mathematically) argued that in this particular instance the error of measurement is not significant and whether or not such 2 point calibration was done is pretty much irrelevant: PH 7.5 being the range of [7.45-7.55] is all you would get and irrespective - it's a low PH reading that needs to be dealt with..
Let me ask you a counter-question: do you calibrate your probe every time you take PH measurement (i.e. every second)? It's the best practive to calibrate your probe to ensure it's most accurate..
Let me pre-empt your answer: why don't you?
How did you transport the water to the lab? How long did it take? I imagine, if the meter is appropriately calibrated there may be some chemical / biological changes going on in solution before your testing it.Hi all,
This morning before lights went on I collected some water from my tank and tested pH in the lab with a lab-grade pH meter.
It says 7.5. I also tested the meter with some calibration solution and it seems about accurate (0.1 difference from the solution at pH 7.0).
With my Sera tests it was looking much more like 8 than 7.5 so I never checked.
This is the situation with my tank:
32.5 g, probably 25g water only.
Closed with lid. Many advised against it but the house is quite old and I don't want to get molds from increased humidity. Also the tank is not completely sealed, there is some space between the lid and the glass and the filter compartment is open. I will try to run the aeration test later this week because I do not have a pH probe at home so I must areate the water outside at work if I manage.
About 1/8th of the volume is occupied by macros
I have 3 fish (YWG, 6 line wrasse, bicolor blenny) and 2 crabs I am worried about (hermit and porcelain crab)
DkH was about 10 last week, I increased it from 8 to 10 using baking soda when I realized it dropped to 8dkh, probably because of macro.
At the moment I have also a cyano/dino outbreak.
How is this possible? I have so much macro in my DT, I should have them absorbing CO2 right?
What I could do in the immediate to increase pH without hurting my animals?
As soon as I found out I turned on the air scrubber in the tank (I use it from time to time in my DT to clean the water). Should I leave it on longer then? maybe all night?
Last edited:
Moving on... I would suggest the OP do some research on how much CO2 it would take to drop 35 ppt (~1.025 SG) seawater, @ say 80 degrees, with an alkalinity in the 7.5 dKh range to a pH of 7.5. I'll suggest that the amount exceeds that found outdoors or even in a reasonable ventilated but closed room. For that reason, I suggest that something else might be wrong like:
1. The probe needs to be properly calibrated;
2. The salinity or alkalinity is not as high as thought;
3. The dissolved organic load is extremely high; or
3. There is some acidic compound being introduced to the tank.
If the tank has sufficient aeration and isn't using a pH lowering contributor such as a CaCO3 reactor, then equilibrium with air CO2 is a good estimate for pH lows.
To have a low pH of 7.5 would require a C02 level of 2800 ppm. Such a high level of CO2 would be very noticable and cause health issues with longer exposure.

If you pH is less than 7.8, your pH meter is probably wrong
Post in thread 'Aquarium pH Distilled' https://www.reef2reef.com/threads/aquarium-ph-distilled.853474/post-9330359
 www.reef2reef.com
www.reef2reef.com
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
Forgot to add... IME, gas exchange is not usually an issue in reasonably operating systems. Even in those with tops. For that reason, I wouldn't include it in the top 5 potential causes of the depressed pH level.
How do you know?
Seems to me the case is exactly the opposite: tanks demonstrably have poor CO2 exchange, hence the entire reason for the day to night pH shift.
Thanks Randy. I suppose I wasn't specific enough and was using some deductive reasoning. I was speaking specifically to the 7.5 pH reading. My reasoning was that almost any circulation that allowed enough gas exchange to keep O2 levels suitable for the livestock would be sufficient to keep pH from being depressed that much... at least without other influences.How do you know?
Seems to me the case is exactly the opposite: tanks demonstrably have poor CO2 exchange, hence the entire reason for the day to night pH shift.
I used a test kit vial closed with sealed lid. It took maybe 2 or 3 hours before I measuredWithout 2 point calibration the slope can be way off.
How did you transport the water to the lab? How long did it take? I imagine, if the meter is appropriately calibrated there may be some chemical / biological changes going on in solution before your testing it.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
If your buffer 7.0 is off by 0.5 and you do a 2 point calibration - this would not save your day.. In my example the 7.1 solution would read 7.6 with single point calibration and 7.58 with 2 point calibration - how is that helping you?
I think you are trying to signifity the importance of multiple reference solutions (of same PH standard) than about multiple point calibration. I would totally agree with this - it's more important to question the validity of your buffer reference solution than "I shall do a 2 point calibration irrespective".
There's no justifiable reason to ever use single point calibration, unless you calibrate at the exact same pH value that you are measuring.
Two point calibration does make it more accurate if one of the buffers is off, in addition to correcting for slope errors in the probe.. And yes, confirming your buffer accuracy will obviously also help.
If your pH 7 buffer is really pH 7.5, and you calibrate your meter with it alone, a real pH 8.2 will read falsely as pH 7.7. In fact, all pH values will read low by 0.5 pH units.
If your pH 7 buffer is really pH 7.5, and you calibrate your meter with it AND a pH 10 buffer that is really pH 10, then
1. A real pH 7.5 will read as pH 7, too low by 0.5 units, same as the single point
2. A real pH 8.2 will read as 7.84, too low by 0.36, less than the single point calibration error
3. A real pH 10 will read as pH 10, with no error from the pH 7 being off
Does two point calibration solve the problem of an off buffer? Of course not. Does it help? yes.
FWIW, I typically did more than two points and included buffers at pH 8.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
Im curious why do ph probes need recalibration? Is it loosing something that needs to be accounted for?
A pH probe works by measuring the electrical potential between two different sides of a glass membrane that is sensitive to H+ in the water. But changes to the glass by other ions, organics and other chemicals impact how the potential changes with H+, hence you need to calibrate it to know for a given electrode, how the electrical signal actually corresponds to H+ changes in the water.
Here's a nice online description of how the membrane works:

Anatomy of pH Electrodes
A typical pH electrode, or pH probe, consists of several unique structures. The principle components are the electrode body, pH-sensitive glass membrane, reference electrode (i.e. reference system), reference electrolyte, and the reference junction that make up pH electrodes.
www.ysi.com
With the glass electrode, a glass membrane is fused on as a pH sensor. This membrane is filled with a buffer solution of known pH (typically pH = 7). This electrode design creates an environment with constant binding of H+ ions on the inside of the glass membrane, while the outside of the glass membrane is exposed to the sample where a variable amount of H+ ions exist. The difference in H+ ions creates a potential that is read versus the stable potential of the reference electrode.
I have 2 cheap Chinese probes (less than $20 each) that have been running for 6 months now. Checked them last week against buffer solutions - they are spot on. I wonder what kind of probes people deal with that they can be 0.5PH off.Im curious why do ph probes need recalibration? Is it loosing something that needs to be accounted for?
Buffers - again, I have a few, and they are within very small tolerance from each other (these are not powder ones - rather liquid pre-made solutions).
One thing that someone else mentioned - inaccuracy in PH measurement is not just coming from the probe, it can be a probe or could also be the board that amplify voltage from probe.
As my PH monitors are DIY'd, I can actually see the returned voltage. Of the 3 boards that are in my controller 2 show the same voltages for 4.0 and 7.0 PH points but one shows completely different voltage pattern (all same make/version by DF Robot). That does not mean that that board is wrong (subject to correct calibration) - but just means the way the board amplify the signal from the probe is generally non-standard.
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
FWIW, here's the rational given by the same company for two or more point calibration vs single point:

3-point calibrations are typically completed when the sample pH conditions are not well understood. Asymmetry and slope are determined for both 2- and 3-point calibrations.
A 1-point calibration will only determine the zero point, not the electrode slope. The range of use of 1-point calibrations is limited and should only be completed with pH 7 buffer. The pH value obtained can be used to compare to previous results but is not an absolute value.
The electrode and calibration container should be rinsed between calibration points with the solution that follows, as any displaced buffer that is carried over can lead to measurement error. For example, rinse with pH 10 buffer if you have finished calibrating to pH 7 and are preparing to calibrate to pH 10. Alternatively, the electrode can be rinsed with DI water and carefully dabbed dry. The calibration container should always be clean. The size of the calibration container typically doesn’t matter, as long as the electrode junction is immersed.

pH Meter Calibration Problems? Check Out These 12 Tips!
You want good pH data. Who doesn’t? You deserve good data, but for good pH data you need to calibrate. You need to calibrate well and calibrate often. pH meter calibration is necessary in order to establish the slope and zero point of the electrode. Since both of these can change over time...
www.ysi.com
. Perform at Least a 2-Point Calibration
It is best to perform at least a 2-point calibration and pH 7 buffer MUST be one of those points. The pH buffers used should differ by at least two pH units and should bracket the expected in-situ pH conditions. Unless the sample is expected to be above pH 7, basic buffers should not be used, as their pH value quickly changes by absorbing CO2.3-point calibrations are typically completed when the sample pH conditions are not well understood. Asymmetry and slope are determined for both 2- and 3-point calibrations.
A 1-point calibration will only determine the zero point, not the electrode slope. The range of use of 1-point calibrations is limited and should only be completed with pH 7 buffer. The pH value obtained can be used to compare to previous results but is not an absolute value.
The electrode and calibration container should be rinsed between calibration points with the solution that follows, as any displaced buffer that is carried over can lead to measurement error. For example, rinse with pH 10 buffer if you have finished calibrating to pH 7 and are preparing to calibrate to pH 10. Alternatively, the electrode can be rinsed with DI water and carefully dabbed dry. The calibration container should always be clean. The size of the calibration container typically doesn’t matter, as long as the electrode junction is immersed.
Looks like your problem to me. Use a larger sample , quicker. Teeny tiny tubes ain't much good.I used a test kit vial closed with sealed lid. It took maybe 2 or 3 hours before I measured
These threads all usually end up with the same suggestions, but in my opinion most of it is meaningless without the use of the simple outside aeration of your sample water and then test ph If ph goes up to what you expect that rules out/minimizes likelihood of a whole lot of useless paths. On the other hand if it it still much lower than it should be at your alkalinity then it almost certainly points to measurement error(either on alkalinity or ph).
My problem is that I have no ph meter at home so 1) I can't use air stone at work 2) I cannot measure ph quickly after collection.These threads all usually end up with the same suggestions, but in my opinion most of it is meaningless without the use of the simple outside aeration of your sample water and then test ph If ph goes up to what you expect that rules out/minimizes likelihood of a whole lot of useless paths. On the other hand if it it still much lower than it should be at your alkalinity then it almost certainly points to measurement error(either on alkalinity or ph).
My test kit has 2 shades of blue very similar if ph is 7.5 or 8. I think it was designed for freshwater
Hi all,
It took a while but I managed to:
re-measured pH: it was 7.6 after few hours lights went on
Check the ID of the dinos in my tank (they appeared when cyano started to disperse): looks like ostreopsis ( )
)
Now, as far as I understood, low pH could be the reason of my present problem with dinos.
I tried to areate the room more often but I am rarely at home and winter is quite cold in Germany. What could I do to raise pH to something like 8? my kH is around 9-10 atm. I don't have corals consuming Mg and Ca so, how much can I raise pH before I hit dKh 12-13 using kalkwasser?
My fish look healthy and my hermit, snails and porcelain crab too but I fear that corals with suffer with pH 7.6 (next month I am buying some Cyphastrea and maybe duncans/hammers etc).
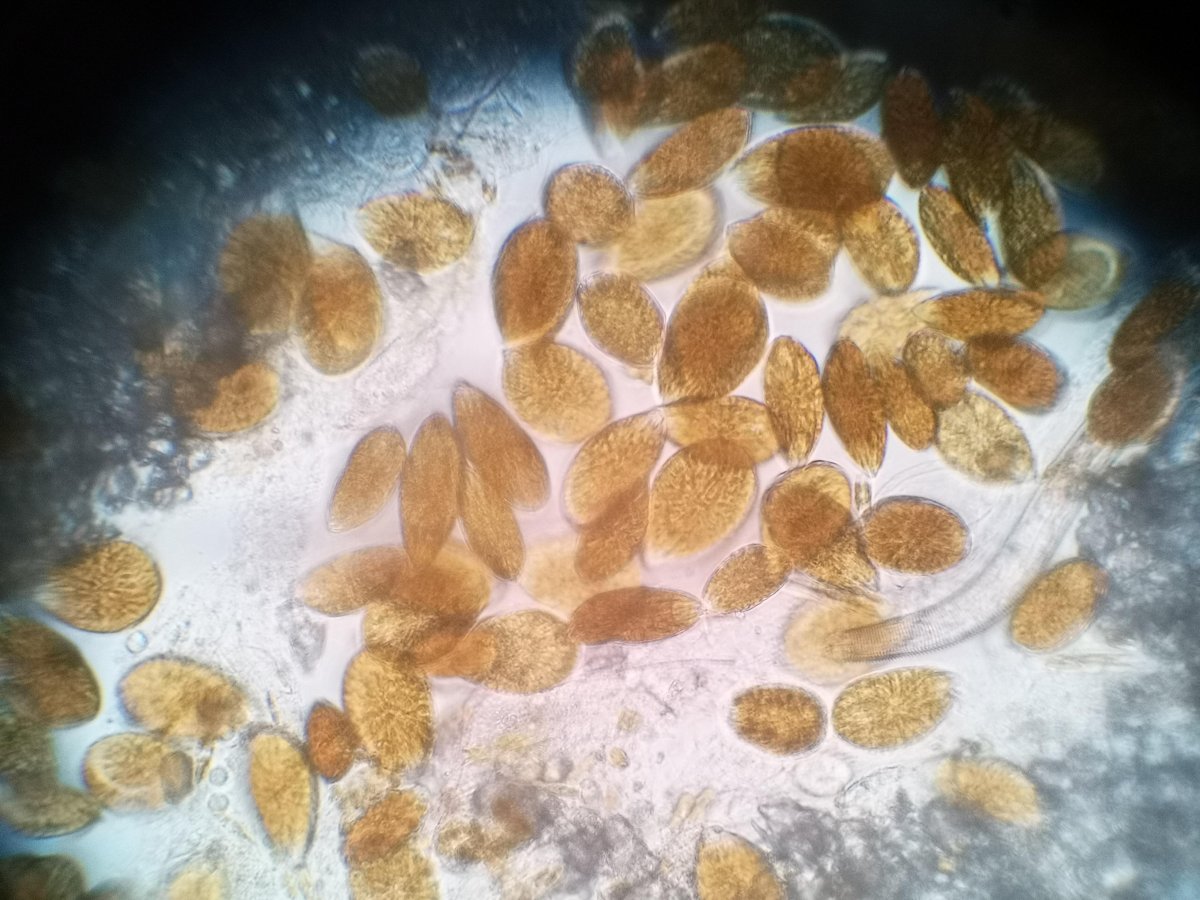
It took a while but I managed to:
re-measured pH: it was 7.6 after few hours lights went on
Check the ID of the dinos in my tank (they appeared when cyano started to disperse): looks like ostreopsis (
Now, as far as I understood, low pH could be the reason of my present problem with dinos.
I tried to areate the room more often but I am rarely at home and winter is quite cold in Germany. What could I do to raise pH to something like 8? my kH is around 9-10 atm. I don't have corals consuming Mg and Ca so, how much can I raise pH before I hit dKh 12-13 using kalkwasser?
My fish look healthy and my hermit, snails and porcelain crab too but I fear that corals with suffer with pH 7.6 (next month I am buying some Cyphastrea and maybe duncans/hammers etc).
A pH of 7.6 is not a realistic measurement unless your tank aeration is poor or your adding low-pH additives.Hi all,
It took a while but I managed to:
re-measured pH: it was 7.6 after few hours lights went on
Check the ID of the dinos in my tank (they appeared when cyano started to disperse): looks like ostreopsis ()
Now, as far as I understood, low pH could be the reason of my present problem with dinos.
I tried to areate the room more often but I am rarely at home and winter is quite cold in Germany. What could I do to raise pH to something like 8? my kH is around 9-10 atm. I don't have corals consuming Mg and Ca so, how much can I raise pH before I hit dKh 12-13 using kalkwasser?
My fish look healthy and my hermit, snails and porcelain crab too but I fear that corals with suffer with pH 7.6 (next month I am buying some Cyphastrea and maybe duncans/hammers etc).

Your indoor CO2 would be at unhealthy levels that would cause you health issues with prolonged exposure.
If you believe your pH is 7.6, then you need to get more water movement on the tank.
A simple cup aeration test can confirm your issue.
Similar threads
- Replies
- 6
- Views
- 151
- Replies
- 27
- Views
- 421
- Replies
- 8
- Views
- 297


















