Thank you,I will give this a shot, I will let you know how it goes.Ok, let's see if this straight forward just based on the error. Can you multiply your water amount by 1.1 please?
So if you were using 750ml, please use 825ml.
Let's see where that gets you.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Xepta AutoBalance reagent
- Thread starter elcapitan1993
- Start date
- Tagged users None
Also you are flushing the reagent out of the unit between switching between DIY and OEM? I just want to make sure there is no carryover.
Yes I flush it every time and I purge the new reagent through for 30 seconds as well just to be safeAlso you are flushing the reagent out of the unit between switching between DIY and OEM? I just want to make sure there is no carryover.
So how is the new ratio looking? Were you able to get closer to OEM?
Just performed the testsSo how is the new ratio looking? Were you able to get closer to OEM?
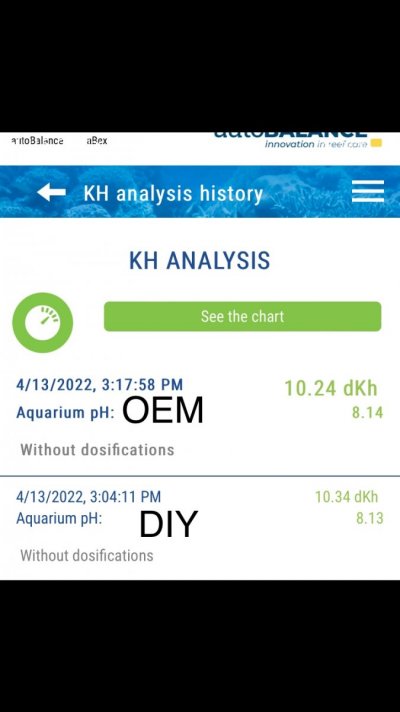
DIY using the 825ml water and 250ml 0.1N HCL: 11.28
OEM REAGENT:11.07
Looks like it was very close this time
With a 00.21 difference based off back to back testing
That is not bad. I think using 810ml water to 250ml 0.1N would be about right.Just performed the tests
DIY using the 825ml water and 250ml 0.1N HCL: 11.28
OEM REAGENT:11.07
Looks like it was very close this time
With a 00.21 difference based off back to back testing
Depending on how accurate your measurements are, a bit of variability may creep in there as well.
No not bad at all, I was wondering how much to tweak it, I’ll try 810ml of water, I’m using a 100ml graduated cylinder to measure it out so it is pretty close but probably not completely perfect, I do have a 100ml and a 1000ml volumetric flask but I don’t know how much it will matter vs the graduated cylinder. I wonder what acid it uses for the OEM reagentThat is not bad. I think using 810ml water to 250ml 0.1N would be about right.
Depending on how accurate your measurements are, a bit of variability may creep in there as well.
I got it within exactly at 0.1 difference of the OEM reagent , I did 810ML of reverse osmosis water and 250 of 0.1N HCL mixed for 20 minutes On a magnetic stirrerThat is not bad. I think using 810ml water to 250ml 0.1N would be about right.
Depending on how accurate your measurements are, a bit of variability may creep in there as well.
Attachments
That is pretty good. So I guess that means a 1:3.24 is about correct.
I think that’s probably as close I am going to get ,well with the equipment I have on hand at least. I’m ok with that margin of error at the moment anyway. What I don’t understand is how their reagent is mixed at 750 to 250 ml and mine doesn’t match that, could they be using sulfuric acid maybe?That is pretty good. So I guess that means a 1:3.24 is about correct.
No it will not be the type of acid, but the normalization they are using. You are using 0.1N, they are using something less than that. It would have been handy if they were using an off the shelf standard like 0.1N, but they seem to not be. Oh well.
If I mixed 750 ml of water to 250 ml of 0.1N HCL what (N) would that make it?
That would make it a bit too strong, with too much acid. The closest of your previous tests was 1:3.24. Mixing 750 ml water and 250 ml HCL would be 1:3If I mixed 750 ml of water to 250 ml of 0.1N HCL what (N) would that make it?
It is a bit of a bummer that they did not use a standard normalization as the basis for their reagent.
If you want to improve your accuracy in mixing, you can get a volumetric flask set off of Amazon that contain various sizes of flasks. For instance, to mix 250 ml of HCL at 1:3.24, you would need a 750, 50 and 10 ml flask for the water. Filling a 10 ml flask would be a chore, so a syringe could replace that.
I was just wondering what moles that would be at that ratio for a different project I’m doing ,That would make it a bit too strong, with too much acid. The closest of your previous tests was 1:3.24. Mixing 750 ml water and 250 ml HCL would be 1:3
It is a bit of a bummer that they did not use a standard normalization as the basis for their reagent.
If you want to improve your accuracy in mixing, you can get a volumetric flask set off of Amazon that contain various sizes of flasks. For instance, to mix 250 ml of HCL at 1:3.24, you would need a 750, 50 and 10 ml flask for the water. Filling a 10 ml flask would be a chore, so a syringe could replace that.
Thankfully I have a set of class A volumetric flasks (was not cheap)
That would be a normalization of 0.025N if mixed at 1:3
Perfect thank you!!That would be a normalization of 0.025N if mixed at 1:3
I have also been talking to xepta support about what molarity they are using, we will see if they are willing to tell me or not.
if I was to use 1.0 m HCL and wanted to make a liter of 0.025 HCL what ratio would I do? I only ask because I have a surplus of 1.0 m HCL I was using to make 0.1n HCL for the kh director, the reagent for the kh director is easier to make but much more expensive with the test to liter ratio
I know to make 0.1n with 1.0 it’s a 9:1 ratio
You would have to mix at 1:30 using 1.0N HCL to get 0.025N. If the final volume is not crucial, you could mix 25ml HCL and 750ml water to get 0.025N.
- Joined
- Jan 15, 2020
- Messages
- 1,521
- Reaction score
- 1,511
How diluted it is has nothing to do with how expensive it is per test. If the sample size is the same then the only difference between 0.1n and 0.025n is that the latter uses 4 times as much reagent which might be preferable to smooth over inaccuracies with the pumps. The amount of actual acid used will be pretty much the samePerfect thank you!!
I have also been talking to xepta support about what molarity they are using, we will see if they are willing to tell me or not.
if I was to use 1.0 m HCL and wanted to make a liter of 0.025 HCL what ratio would I do? I only ask because I have a surplus of 1.0 m HCL I was using to make 0.1n HCL for the kh director, the reagent for the kh director is easier to make but much more expensive with the test to liter ratio
I know to make 0.1n with 1.0 it’s a 9:1 ratio
MYou would have to mix at 1:30 using 1.0N HCL to get 0.025N. If the final volume is not crucial, you could mix 25ml HCL and 750ml water to get 0.025N.
i am not saying that I’m saying getting a liter of 1.0 m and 0.1 m is the same price but you technically get more liters of the diluted reagent out of itHow diluted it is has nothing to do with how expensive it is per test. If the sample size is the same then the only difference between 0.1n and 0.025n is that the latter uses 4 times as much reagent which might be preferable to smooth over inaccuracies with the pumps. The amount of actual acid used will be pretty much the same
I dicided to give the DIY reagent a go tonight by measuring by weight rather than using measuring cylinders.That is pretty good. So I guess that means a 1:3.24 is about correct.
I also decided to mix it on a large scale but still following the ratio given above.
5 litres of 0.1N mixed with 16.2 litres of Rodi water. Totalling 21.2 litres of reagent. Unfortunately I think the ratio given above isn't completely accurate for larger scale batches.
Xepta reagent test: 8.00dkh
Diy reagent test: 8.99dkh
Similar threads
- Replies
- 55
- Views
- 1,155
- Replies
- 18
- Views
- 583
- Replies
- 4
- Views
- 479