Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Spirulina what is it, and what does it look like
- Thread starter twilliard
- Start date
- Tagged users None
Twillard, we may be engaging in a game of semantics, but the only reference to spirulina being an algae that I can find is when they call it a Blue-Green Algae (which is an older name/synonym for cyanobacteria. Unfortunately, the use of BGA is so firmly ensconced in our vocabulary, that it still gets used today). All other references that I can find call it a cyanobacteria.
It's been 40 years since I studied microbiology in university, and haven't used it since. But why it doesn't respond to H2O2 the same way as our regular reef cyano probably has to do with its differing structure and composition. I've read a number of studies on spirulina and how it differs from other strains of Cyanobacterium, so to me it would make sense that it would react differently to H2O2.
Nevertheless, a review of a number of articles on Spirulina indicates that its growth can be nitrogen limited in some FW species and carbon dioxide limited in other species.
"Microalgae and cyanobacteria can contribute to the reduction of atmospheric carbon dioxide by using this gas as carbon source. We cultivated the Scenedesmus obliquus and Spirulina sp. at 30 °C in a temperature-controlled three-stage serial tubular photobioreactor and determined the resistance of these organisms to limitation and excess of carbon dioxide and the capacity of the system to fix this greenhouse gas. After 5 days of cultivation under conditions of carbon limitation both organisms showed cell death. Spirulina sp. presenting better results for all parameters than S. obliquus."
"Natural populations of the cyanobacteria Spirulina species and Oscillatoria species obtained from Israeli fishponds were limited in growth by nitrogen availability in summer."
It's not much, but it might help in developing a method to control this BGA/cyanobacterium.
I am trying to help. Honest
It's been 40 years since I studied microbiology in university, and haven't used it since. But why it doesn't respond to H2O2 the same way as our regular reef cyano probably has to do with its differing structure and composition. I've read a number of studies on spirulina and how it differs from other strains of Cyanobacterium, so to me it would make sense that it would react differently to H2O2.
Nevertheless, a review of a number of articles on Spirulina indicates that its growth can be nitrogen limited in some FW species and carbon dioxide limited in other species.
"Microalgae and cyanobacteria can contribute to the reduction of atmospheric carbon dioxide by using this gas as carbon source. We cultivated the Scenedesmus obliquus and Spirulina sp. at 30 °C in a temperature-controlled three-stage serial tubular photobioreactor and determined the resistance of these organisms to limitation and excess of carbon dioxide and the capacity of the system to fix this greenhouse gas. After 5 days of cultivation under conditions of carbon limitation both organisms showed cell death. Spirulina sp. presenting better results for all parameters than S. obliquus."
"Natural populations of the cyanobacteria Spirulina species and Oscillatoria species obtained from Israeli fishponds were limited in growth by nitrogen availability in summer."
It's not much, but it might help in developing a method to control this BGA/cyanobacterium.
I am trying to help. Honest
And how are you doing this?
If I knew I would get rid of it. But it's starting to get kinda bad.
jsker
Reefing is all about the adventure
View Badges
Staff member
Super Moderator
Excellence Award
Reef Tank 365
Article Contributor
Hospitality Award
Ocala Reef Club Member
R2R Secret Santa 2023
My Tank Thread
I am dealing with the same issue. I am giving the H2O2 treatment till Sunday then I am going for the nuclear option and then I will play around with H2O2 as maitance.If I knew I would get rid of it. But it's starting to get kinda bad.
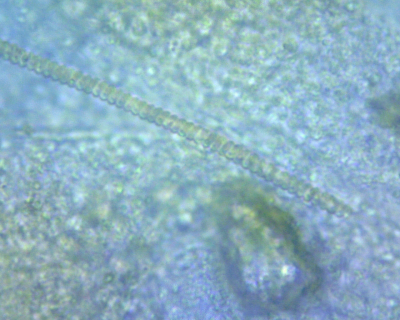
Its difficult to see - are the cell walls equally thick? Looks every cell the same in one string or are there cells that is a little bit larger surrounded of thicker cell walls? The strain you cultivate – does it build matts on the substrate or is it only floating in the surface?
Sincerely Lasse
It's hard to explain this my friend. It's 1 cell that is in a spiral shape of equal diameterIts difficult to see - are the cell walls equally thick? Looks every cell the same in one string or are there cells that is a little bit larger surrounded of thicker cell walls? The strain you cultivate – does it build matts on the substrate or is it only floating in the surface?
Sincerely Lasse
The size of these cells are hard to capture at 2500 power (the max for my scope) The background you see is of an unidentified algae (I might have a contaminated test
Also these strands can be extremely long. These from what I can see do not have a cell structure as our common tank cyanobacteria.
The strain I have adheres to surfaces. I have not been able to see and free cells in the water column of my beaker.
Google heterocyst - and you understand what I´m out for.
Sincerely Lasse
Sincerely Lasse
These cells are not found within the strands of my spirulina cultureGoogle heterocyst - and you understand what I´m out for.
Sincerely Lasse
I have yet to find the "end" of a spirulina strand. Still looking for this.
I have seen these however in previous cyanobacteria cultures
@twilliard
Have you seen heterocyst in benthic cyanobacteria who create mats in reef aquariums? Is it common? It´s very interesting if we have strains of cyano bacteria in our aquariums that can create this special cell types. A heterocyst is able to fix nitrogen from nitrogen gas – i.e. it is not nitrogen limited.
My standard procedure when I detect red mats in my aquarium is to try to take it away as often as possible and to dose nitrate. In 90 % of the cases it works. The most common thought is that these mats do not have any heterocyst but I have a theory that they create anaerobic conditions below the math and co works with nitrogen fixating bacteria. If it is this way – they probably also are able to extract phosphorus from the substrate and also become not phosphorus limited. Anaerobic conditions and lack of nitrate create sulphur hydrogen – and sulphur hydrogen are able to release bound phosphorus to the water column.
A study from Swedish and Danish lakes has shown that cyanobacteria blooms normally do not happen in lakes with nitrate levels around 2 ppm (and over) even if the bottom layer is anaerobic. The studies has shown pathways there cyano bacteria goes from the water column during the night and down to the sediment – pick up phosphorus and during the day phosphorus enriched cyano bactreria travel up in the light and start growing! - but it demands anaerobic conditions above the sediment together with production of sulphur hydrogen. If there is nitrate in the water - the production of sulphur hydrogen is very limited even if it is stagnant bottom water
If there is strains that have heterocyst - the dosing of nitrate is probably more important (if you got just that strain?)
By the way – where does dinos goes during night? I guess down in the gravel – what do they do in the gravel? Maybe picking up phophorus?
Sincerely Lasse
Have you seen heterocyst in benthic cyanobacteria who create mats in reef aquariums? Is it common? It´s very interesting if we have strains of cyano bacteria in our aquariums that can create this special cell types. A heterocyst is able to fix nitrogen from nitrogen gas – i.e. it is not nitrogen limited.
My standard procedure when I detect red mats in my aquarium is to try to take it away as often as possible and to dose nitrate. In 90 % of the cases it works. The most common thought is that these mats do not have any heterocyst but I have a theory that they create anaerobic conditions below the math and co works with nitrogen fixating bacteria. If it is this way – they probably also are able to extract phosphorus from the substrate and also become not phosphorus limited. Anaerobic conditions and lack of nitrate create sulphur hydrogen – and sulphur hydrogen are able to release bound phosphorus to the water column.
A study from Swedish and Danish lakes has shown that cyanobacteria blooms normally do not happen in lakes with nitrate levels around 2 ppm (and over) even if the bottom layer is anaerobic. The studies has shown pathways there cyano bacteria goes from the water column during the night and down to the sediment – pick up phosphorus and during the day phosphorus enriched cyano bactreria travel up in the light and start growing! - but it demands anaerobic conditions above the sediment together with production of sulphur hydrogen. If there is nitrate in the water - the production of sulphur hydrogen is very limited even if it is stagnant bottom water
If there is strains that have heterocyst - the dosing of nitrate is probably more important (if you got just that strain?)
By the way – where does dinos goes during night? I guess down in the gravel – what do they do in the gravel? Maybe picking up phophorus?
Sincerely Lasse
Oh my we think alike!@twilliard
Have you seen heterocyst in benthic cyanobacteria who create mats in reef aquariums? Is it common? It´s very interesting if we have strains of cyano bacteria in our aquariums that can create this special cell types. A heterocyst is able to fix nitrogen from nitrogen gas – i.e. it is not nitrogen limited.
My standard procedure when I detect red mats in my aquarium is to try to take it away as often as possible and to dose nitrate. In 90 % of the cases it works. The most common thought is that these mats do not have any heterocyst but I have a theory that they create anaerobic conditions below the math and co works with nitrogen fixating bacteria. If it is this way – they probably also are able to extract phosphorus from the substrate and also become not phosphorus limited. Anaerobic conditions and lack of nitrate create sulphur hydrogen – and sulphur hydrogen are able to release bound phosphorus to the water column.
A study from Swedish and Danish lakes has shown that cyanobacteria blooms normally do not happen in lakes with nitrate levels around 2 ppm (and over) even if the bottom layer is anaerobic. The studies has shown pathways there cyano bacteria goes from the water column during the night and down to the sediment – pick up phosphorus and during the day phosphorus enriched cyano bactreria travel up in the light and start growing! - but it demands anaerobic conditions above the sediment together with production of sulphur hydrogen. If there is nitrate in the water - the production of sulphur hydrogen is very limited even if it is stagnant bottom water
If there is strains that have heterocyst - the dosing of nitrate is probably more important (if you got just that strain?)
By the way – where does dinos goes during night? I guess down in the gravel – what do they do in the gravel? Maybe picking up phophorus?
Sincerely Lasse
You are absolutely correct on your thoughts.
Sorry I don't have a lot of time to respond but I would love to see a PM from you.
I get hammered all day!
@twilliard
Ha
My standard procedure when I detect red mats in my aquarium is to try to take it away as often as possible and to dose nitrate.
When dosing nitrates, What are you using? And when doing that i'm guessing your taking out pretty much all media?
@scfurse77
I start dose as soon as I see the red mats start growing. I try to remove them through siphoning them out as soon as possible. If I´m short of hermit crabs - I get some more. I dose for a week or so and up to 2-3 ppm daily. I do not measure nitrate – I do not trust the tests!
The reason for this maintains is to try to reduce the cyano biomass, try to avoid the mats (I believe that the mats and what happens below them is the key) and try to take away organic matter below the mats. (by the way – this is the only occasion when I touch my gravel)
The nitrate adding has two reasons. Give other algae a chance to compete and minimize the possibility for hydrogen sulphide production below the mats.
This normally works in mine aquariums – is not sure it works everywhere because of different strains and species of cyano bacteria and different ways of general maintenance.
I use potassium nitrate or sodium nitrate from the grocery store
@twilliard PM on its way
Sincerely Lasse
I start dose as soon as I see the red mats start growing. I try to remove them through siphoning them out as soon as possible. If I´m short of hermit crabs - I get some more. I dose for a week or so and up to 2-3 ppm daily. I do not measure nitrate – I do not trust the tests!
The reason for this maintains is to try to reduce the cyano biomass, try to avoid the mats (I believe that the mats and what happens below them is the key) and try to take away organic matter below the mats. (by the way – this is the only occasion when I touch my gravel)
The nitrate adding has two reasons. Give other algae a chance to compete and minimize the possibility for hydrogen sulphide production below the mats.
This normally works in mine aquariums – is not sure it works everywhere because of different strains and species of cyano bacteria and different ways of general maintenance.
I use potassium nitrate or sodium nitrate from the grocery store
@twilliard PM on its way
Sincerely Lasse
Thank you @cowboy
Few people know that nitrate concentration in the water (and sediment/gravel) prevent much of the formations of H2S. The common use of organic carbon will also speed up the denitrification rate - and depletion of nitrate. I forgot to mention that I also stop dosing organic carbon if I get red mats
Sincerely Lasse
Few people know that nitrate concentration in the water (and sediment/gravel) prevent much of the formations of H2S. The common use of organic carbon will also speed up the denitrification rate - and depletion of nitrate. I forgot to mention that I also stop dosing organic carbon if I get red mats
Sincerely Lasse
- Joined
- Dec 22, 2015
- Messages
- 796
- Reaction score
- 685
Nitrates even in the former of nitrogen help with the management of H2S. With my work with @twilliard I did notice some H2S at least 10ppm underneath the mats, But the only way I have to measure it is a high low meter 10ppm and 20ppm. It wasn't until I read your post on the use of nitrate that it clicked to my aquarium. It's kinda like that bright light bulb that it work 10000 ft down why won't it work in a 2 ft deep aquarium. You will have to excuse me my chemistry in the oil and gas industry is a bit advanced but in the aquarium industry I tend to overlook the obvious.
I got my knowledge in this case from work at a waste water plant 20 years ago  It took some time befor it even click for me
It took some time befor it even click for me 
Your measurement result underneath the mats was interesting. Is there any possibilities to do the same measurements a day or more after an addition of nitrate or after an injection of nitrate under the mats?
Sincerely Lasse
Your measurement result underneath the mats was interesting. Is there any possibilities to do the same measurements a day or more after an addition of nitrate or after an injection of nitrate under the mats?
Sincerely Lasse
- Joined
- Dec 22, 2015
- Messages
- 796
- Reaction score
- 685
Unfortunately I don't have the equipment for that the only reason I was able to get the reading before was I clipped my H2S monitor to the bucket while preforming a water change.
What are you using to dose your nitrates I'm going to start a massive culture of cyano and spirulina. I would like to test this and document.
What are you using to dose your nitrates I'm going to start a massive culture of cyano and spirulina. I would like to test this and document.
I use sodium nitrate or potassium nitrate from the grocery store. I do a bulk solution – 40 grams of KNO3 or NaNO3 to 460 grams of water. Of this bulk solution – 1 ml give a rise of around 0,5 ppm to 100 l (as nitrate). It is not exactly – I know – but close enough. I have notice that I need to dose so much that I reach 3 - 5 ppm each day for a period of a week And stop organic carbon dosage.
The reason why I sometimes use NaNO3 instead of KNO3 is some concerns not to have a to high K content in my aquarium. I and a friend has a run for a mystic kill of our fishes. They had a dying pattern that I never have seen before (and I have seen a lot) . At the same time – Triton test show very high potassium levels (around 600 ppm). Two occasions are not a prove but an indication – I think. The high concentration was probably caused of an over dosage of K in order to get a good coral health – at least in my case. By the way – the corals have never been better – but the fishes die.
Please come back with your results - I´m very interested to know the outcome
Sincerely Lasse
The reason why I sometimes use NaNO3 instead of KNO3 is some concerns not to have a to high K content in my aquarium. I and a friend has a run for a mystic kill of our fishes. They had a dying pattern that I never have seen before (and I have seen a lot) . At the same time – Triton test show very high potassium levels (around 600 ppm). Two occasions are not a prove but an indication – I think. The high concentration was probably caused of an over dosage of K in order to get a good coral health – at least in my case. By the way – the corals have never been better – but the fishes die.
Please come back with your results - I´m very interested to know the outcome
Sincerely Lasse
Similar threads
- Replies
- 20
- Views
- 673
- Replies
- 3
- Views
- 146
- Replies
- 20
- Views
- 273
TOP 10 Trending Threads
- Replies
- 36
- Views
- 547
- Replies
- 81
- Views
- 888
- Replies
- 30
- Views
- 489
- Replies
- 44
- Views
- 632
- Replies
- 46
- Views
- 715
- Replies
- 20
- Views
- 204
- Replies
- 68
- Views
- 639
- Replies
- 30
- Views
- 684