Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
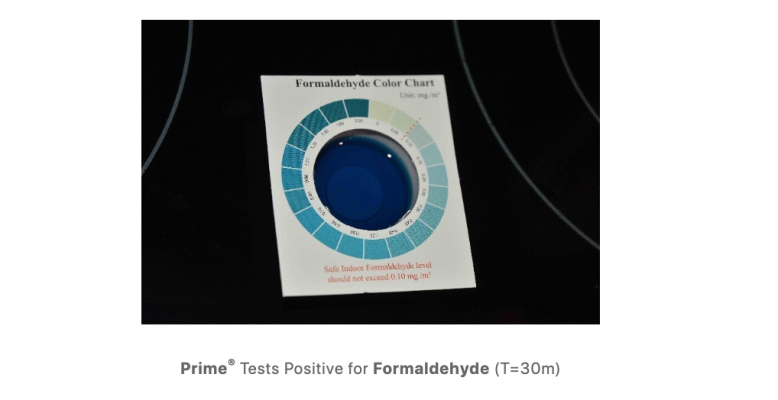
I’m just reporting what happened over the past few days
Nitrate was the same color as it has been - barely above zero - and I’ve been tracking this for weeks because I’m having a low nutrient issue
My ammonia had a sudden spike which was confirmed over 3 test kits, I dosed prime aggressively, now it’s near 0 with no other change in other params
Maybe the tank handled it in one day with no detectable change in nitrate or nitrite, or maybe Prime had some beneficial effect
You seem to be aggressively dismissing this because it goes against the running hypothesis - I personally don’t have a dog in the fight and I’m just reporting what I’m seeing first hand
(This ammonia spike is what drove me to find this thread in the first place)
I don’t understand, I’m not dismissing any observation that you made and your assertion that I am indicates that you are misunderstanding what I am saying.
Simply put, every observation you made is perfectly consistent with Prime doing nothing. It is also consistent with Prime being useful to detoxify ammonia.
Thus, none of your observations bear on whether it did anything useful or not.