Just checked my 60g dt ammonia with hanna is 0.05. So its not the checker. I will check the prepared water tomorrow. I agree I know I need to do a water change just keep hoping it will trend down and it doesnt.It never hurts to check your instrument/kit against another system, especially if that system has tests results from a different instrument. It may not tell you which one is correct if they are different. If they are close it can give you some peace of mind.
Even bottled bac is going to take a day or three to do its thing, depending on how out of balance the system is and how much bacteria is in the bottle compared to the load it has to multiply and process.
When you scoop and move sand, the shear and crushing forces will kill a tremendous amount of the macro fauna living in it. If there were nems, crabs, isopods, mini stars, etc. mixed in, then they were likely damaged and/or ground into the sand as it was removed and replaced.
I would be considering a water change even if what is left is looking a bit better.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Mini cycle
- Thread starter alicia24
- Start date
- Tagged users None
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
It will trend down, but your guess is as good as mine as to what it will peak at or when.Just checked my 60g dt ammonia with hanna is 0.05. So its not the checker. I will check the prepared water tomorrow. I agree I know I need to do a water change just keep hoping it will trend down and it doesnt.
You may still want to check your change water, but honestly if it is the same salt and water that you use for the other tank, then there is no need unless the volume that you made for this new tank is significantly more and far apart from the last time that you made water.
Lots of folk make this assumption. However, there are other things to be considered. For example, how much ammonia was previously being added to the tank in food, what fraction of this was taken up by photosynthetic organisms compared to nitrifying bacteria, what fraction of this was taken up by heterotrophic bacteria (especially if the tank was carbon dosed). The answer to these questions are likely unknowable, all we can do is deal with the consequences.I thought I mature reef could handle 2ppm in a day so I am also very confused and frustrated.
It is the same salt and water I use for the other tank. I did a water change in the 60g 2 weeks and it was only 20% change this tank was like 60-70%.It will trend down, but your guess is as good as mine as to what it will peak at or when.
You may still want to check your change water, but honestly if it is the same salt and water that you use for the other tank, then there is no need unless the volume that you made for this new tank is significantly more and far apart from the last time that you made water.
Oh my ok thank you for clearing that up!Lots of folk make this assumption. However, there are other things to be considered. For example, how much ammonia was previously being added to the tank in food, what fraction of this was taken up by photosynthetic organisms compared to nitrifying bacteria, what fraction of this was taken up by heterotrophic bacteria (especially if the tank was carbon dosed). The answer to these questions are likely unknowable, all we can do is deal with the consequences.
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
Exactly - but I really only know one person who preaches that "assumption" as fact -- calls it "new cycling science" and demeans anybody and everybody who calls it the nonsense that it is.Lots of folk make this assumption. However, there are other things to be considered. For example, how much ammonia was previously being added to the tank in food, what fraction of this was taken up by photosynthetic organisms compared to nitrifying bacteria, what fraction of this was taken up by heterotrophic bacteria (especially if the tank was carbon dosed). The answer to these questions are likely unknowable, all we can do is deal with the consequences.
Great- I would not waste the reagent if you are running low. Your numbers are rising in one tank and not the other so we can pretty much rule out the source water.It is the same salt and water I use for the other tank. I did a water change in the 60g 2 weeks and it was only 20% change this tank was like 60-70%.
- Joined
- Sep 21, 2018
- Messages
- 6,708
- Reaction score
- 7,189
@Dan_P
Is there any way you could take a calibrated accurate seneye unit and put it near your sandbed with no disturbance to recalibrate a baseline then just do one stick stir tiny right near it. Curious if nh3 spikes.
I need @taricha to jog my memory of out many discussions and experiments on sand beds. In short, they are complicated ecosystems.
If you carefully remove water from the sand (pore water) without mixing it with water from above the sand bed, the water between the sand grains contains ammonia and a higher concentration of phosphate. Dissolved oxygen is definitely lower by several ppm. i think the alkalinity and pH are also different from the water above the sand bed. The total amount of pore water compered to the total amount of aquarium water is small and alone would not cause a large ammonia spike. A sudden release and subsequent digestion of organic matter from the sand when stirred up might cause ammonia to rise (I have not done this).
Yeah, the Seneye would be a device to detect ammonia in the sand. Stirring the sand and catching a whiff of ammonia might work. The “might” qualification is based on not mixing too much above sand water with the pore water. The merit in this idea for me is that the sand bed as an ammonia generator could be part of or the entire explanation for why nuisance algae grow immense populations on sand (why they grow on rock might need a further elaboration).
Here is why it is tricky for me to think about this. For one thing, it is probably easier than we think to create areas of anaerobic sand where nitrification does not occur and ammonia accumulates. Secondly, nitrifiers can live “next door” to anaerobic biofilms where there is sufficient oxygen and plenty of CO2 to do their thing. Thirdly, sufficient light penetrates the sand bed to support photosynthesis millimeters down, maybe deeper. Trying to think about the nitrogen economy and how ammonia is processed typically leaves me with no insights. @taricha I think has done some experiments to tease out the proportions of ammonia processed via photosynthetic organisms and nitrifyers - or at least had some clever ideas on the subject. It’s been years since.Curious if a common reef tank sandbed has unused ammonia, as if a reef sandbed is always anaerobic and would allow no photosynthetics or nitrifiers to be present alongside the claimed shale reservoirs of ammonia to simply keep them in check even if originating from protein decomposition in the sand
We've all spent a good deal focusing on the bacteria. Her system above is unique in its aging and maturity. I bet even if the bac are dead that much inclusive photosynthetic material on those types of rocks, those rocks are aged close to or beyond ten years i can see in the benthic life quality- pulls ammonia fast
Their plant loading alone commands ammonia, fast. So if that seneye spikes legit, I'll be left confounded for eight days in preponderance
But if you report a resolve rate for the spike at 1-3 minutes vs a ten hour reduction delay, that'll apply here bigtime in scale testing.
Lots and lots of information missing to have a good feeling about what should have happened. Light level and water motion can play a huge role in determining just how well the photosynthetic organisms will handle a relatively quick rise in ammonia. Depleted trace elements (not all of them maybe just Fe and Mo) could choke ammonia removal rate.
Yes, old rocks might be well populated with ammonia consumers after ten years, In an aquarium where water flow is relatively poor, the rock surfaces might have thick biofilms that might act as a barrier to bringing ammonia and CO2. Biofilms are stick and all sorts of stuff sticks to them, adding more resistance to the influx of ammonia. Sometimes old isn’t so good
I think death and decay of organisms rather than detritus accumulation is the likely cause of ammonia spikes, especially long duration ones. In an aquarium, bacteria and other larger organisms are not going to let food accumulate. I picture their appetites like those of my dogs: insatiable. If more food becomes available, the more of them there are. The aquarium sand bed probably does not have the intensity of organic matter raining down on it like that in the ocean or on a reef.Proximity of the probe to the sand when you light stir makes up for dilution in my opinion. Heck, turn off pumps for a sec and let it bake a sec if we need a stress test in scale
I would have never considered photosynthetic command from micro benthic plant communities before seeing yours and Tarichas super good works. If reef tank sandbeds have ammonia and you have considered any alternate compounds in detritus, you guys' testing for detritus has shown relative inertness so where would loads of ammonia come from, I'll take that to heart and try to work it into my understanding of stratification levels in reef tanks.
That's important data for tank transferers/ where are the shale deposits
I will give your experiment more thought.
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
I think people assume "new sand" on a move is simply to eliminate detritus but the amount of death (and resulting decay) in a tank move is extremely underestimated, especially one where the sand is moved.I think death and decay of organisms rather than detritus accumulation is the likely cause of ammonia spikes, especially long duration ones.
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
This would have been that calm place if it were not for the pages of rhetoric that you posted and have now conveniently walked away instead of admitting error.That's just so valuable. I'll be restudying that in a calm place in the park on my next bike ride. Thank you for that Dan for sure
Below are just a few of the many examples in just this thread.
Are you going to stand corrected?
Last edited:
- Joined
- Apr 20, 2019
- Messages
- 14,376
- Reaction score
- 22,058
This would have been that calm place if it were not for the pages of rhetoric that you posted and have now conveniently walked away instead of admitting error.
Below are just a few of the many examples in just this thread.





Are you going to stand corrected?
It's incredible that you and a few others continue to hijack threads by bickering with other posters instead of just focusing on your own advice and the OP. Its always the same argument over and over and over for pages and pages. You seem to have a particular vendetta with Brandon. Learn to move on.
This is how I like it
I haven't seen any trolling so far
Sometimes I spot check, but never Erin.
She could check ignore on me but she never will. Like Aerosmith in the 70s had hangers on, garf, Erin, bean animal like to follow me from Cincinnati to LA so they can keep slashing my van tires and trying to steal my les Paul.
It never ends, they live for controversy not reef outcomes.
I'm in this thread to get input that shapes my work threads and keeps my results for others safe.
By using ignore, readers can change the thread they see into only those types of posters. Anyone who wants to ignore and not read my input could have done so in 2018. Garf and Erin have been on block since then (her old avatar name, I included this new one too for obvious reasons)
Dans future test is the awaited input I'll be so happy to see the graphs and especially the resolve rates.
This entire thread is just a resolve rate debate. Nobody was ever going to actually test their perspective in a helpful manner but I knew Dan could. We're lucky he's looking into it.

I haven't seen any trolling so far
Sometimes I spot check, but never Erin.
She could check ignore on me but she never will. Like Aerosmith in the 70s had hangers on, garf, Erin, bean animal like to follow me from Cincinnati to LA so they can keep slashing my van tires and trying to steal my les Paul.
It never ends, they live for controversy not reef outcomes.
I'm in this thread to get input that shapes my work threads and keeps my results for others safe.
By using ignore, readers can change the thread they see into only those types of posters. Anyone who wants to ignore and not read my input could have done so in 2018. Garf and Erin have been on block since then (her old avatar name, I included this new one too for obvious reasons)
Dans future test is the awaited input I'll be so happy to see the graphs and especially the resolve rates.
This entire thread is just a resolve rate debate. Nobody was ever going to actually test their perspective in a helpful manner but I knew Dan could. We're lucky he's looking into it.
Last edited:
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
And you appear to have a particular vendetta with me. The advice works both ways. You showed up simply to levy a personal attack. Learn to move on.You seem to have a particular vendetta with Brandon. Learn to move on.
Last edited:
- Joined
- Jul 16, 2009
- Messages
- 3,244
- Reaction score
- 4,892
Good luck with the tank. I am going to step away from the thread to avoid further back and forth.It is the same salt and water I use for the other tank. I did a water change in the 60g 2 weeks and it was only 20% change this tank was like 60-70%.
Last edited:
Thank you so much!! Really appreciate the help!Good luck with the tank. I am going to step away from the thread to avoid further back and forth.
- Joined
- Apr 20, 2019
- Messages
- 14,376
- Reaction score
- 22,058
And you appear to have a particular vendetta with me. The advice works both ways. Learn to move on.
I would love it if I could hop into a thread and not see a line by line breakdown of how you disagree with Brandon or someone else's OPINION. Unfortunately you have been doing this in many different threads.
You disagree with Brandon's philosophy/methods/communication style. Got it. Every thread does not have to devolve into a referendum between you and whomever you disagree with. It detracts from the actual issues.
So many simple questions that might take a few responses to resolve often turn into 5 pages of back and forth bickering that in the end solves nothing but massaging ego's. Rinse, repeat.
This means that you have around 0.07 in free NH3. Its an alert situation. Not panic but alert - you need to consider what to doPh is still low at 7.8. Skimmer is on. Ammonia is 2.23
The low pH indicate that you have a lot of heterotrophic bacteria working releasing both CO2 and NH3/NH4. I think that the MB7 addition have worsening the situation
This is a little tricky situation IMO because whatever you do - you have your end in the back.
If you rise your aeration - you will aerate out NH3 but also rise the pH - more NH3 into the water. If you go down with the aeration there is risk that anaerobic pockets will be formed.
If I was in your situation I would probably do like this
Let the pH be low (7.8) not doing any more in order to rise it for the moment.
Note - there is a risk with this tip
There is a trick to keep the pH low (hence minimize the risk for forming toxic NH3) but you should only do it if you are sure of what you are doing. Its to add carbonated water. Here i Sweden home carbonators are common and I have used small amount of water from them in order to lower the pH sometimes. I´m sure you can use carbonate water from shops too if it is pure without essences. You need to test with small amount - its powerful.
I would prepare a WC but do it in the 60 gallon first.
Stir the upper layer of 60 gallons sand a little and suck out water in buckets - keep the water (and the mulm) for the problematic aquarium
Fill up the 60 Gallon with new water.
Suck out water from the problematic aquarium - try to get some mulm from it too - discharge this water and mulm
Fill up the problematic aquarium with the wastewater you keep from your 60 G - with the mulm from the 60 G
You can repeat this on daily base with smaller amount of WC if you have time and resources
In this way you get both better water (lower in NH3/NH4) and nitrification bacteria from the working aquarium and get out water with high NH3/NH4 and mulm with mostly heterotrophic bacteria.
I would also put in an internal filter that use foam as filter media - maybe first in the working aquaria (to seed it) and move it after some days to the problematic aquaria.
IMO - you can trust Hanna ammonia checker - I use the same method with my Hanna Marine Master.
All aquarium is different - they are all different ecosystem with different micro and macro biota - therefore is judgement of a problem in a certain aquarium build on "working threads" from other aquariums worthless IMO and also dangerous. Every problem in every aquarium have to be analyzed and remedied on the basis of its individual conditions IMO.
There is however basic chemical, biological and ecological principles that must be considered when you analyze a problem and many posters in this thread have try to give their advises based on that more than outcome from cases there you do not know the full circumstances. Its like a book that I read that describe the life of a delivery driver that every night in full speed passed a bridge that was surrounded of fog - it was impossible to see what was on the bridge. The book ends with the line - this night had a truck get engine stop in the middle of the bridge
Sincerely Lasse
Last edited:
Thank you! What is "mulm?" I actually have to do a wc in the 60 so I'm glad you mentioned that I would have just tossed the water. Thanks! Lowering the ph I could just turn the skimmer off?This means that you have around 0.07 in free NH3. Its an alert situation. Not panic but alert - you need to consider what to do
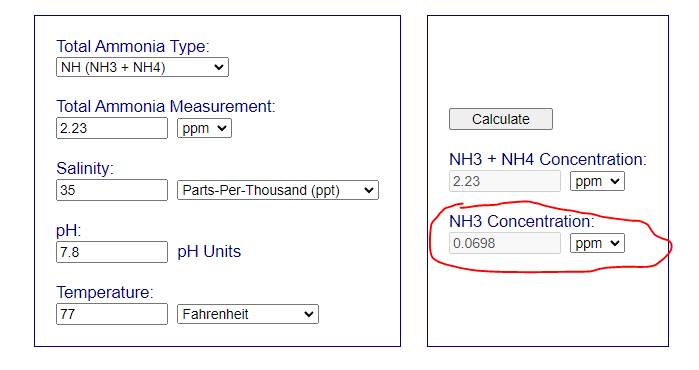
The low pH indicate that you have a lot of heterotrophic bacteria working releasing both CO2 and NH3/NH4. I think that the MB7 addition have worsening the situation
This is a little tricky situation IMO because whatever you do - you have your end in the back.
If you rise your aeration - you will aerate out NH3 but also rise the pH - more NH3 into the water. If you go down with the aeration there is risk that anaerobic pockets will be formed.
If I was in your situation I would probably do like this
Let the pH be low (7.8) not doing any more in order to rise it for the moment.
Note - there is a risk with this tip
There is a trick to keep the pH low (hence minimize the risk for forming toxic NH3) but you should only do it if you are sure of what you are doing. Its to add carbonated water. Here i Sweden home carbonators are common and I have used small amount of water from them in order to lower the pH sometimes. I´m sure you can use carbonate water from shops too if it is pure without essences. You need to test with small amount - its powerful.
I would prepare a WC but do it in the 60 gallon first.
Stir the upper layer of 60 gallons sand a little and suck out water in buckets - keep the water (and the mulm) for the problematic aquarium
Fill up the 60 Gallon with new water.
Suck out water from the problematic aquarium - try to get some mulm from it too - discharge this water and mulm
Fill up the problematic aquarium with the wastewater you keep from your 60 G - with the mulm from the 60 G
You can repeat this on daily base with smaller amount of WC if you have time and resources
In this way you get both better water (lower in NH3/NH4) and nitrification bacteria from the working aquarium and get out water with high NH3/NH4 and mulm with mostly heterotrophic bacteria.
I would also put in an internal filter that use foam as filter media - maybe first in the working aquaria (to seed it) and move it after some days to the problematic aquaria.
IMO - you can trust Hanna ammonia checker - I use the same method with my Hanna Marine Master.
All aquarium is different - they are all different ecosystem with different micro and macro biota - therefore is judgement of a problem in a certain aquarium build on "working threads" from other aquariums worthless IMO and also dangerous. Every problem in every aquarium have to be analyzed and remedied on the basis of its individual conditions IMO.
There is however basic chemical, biological and ecological principles that must be considered when you analyze a problem and many posters in this thread have try to give their advises based on that more than outcome from cases there you do not know the full circumstances. Its like a book that I read that describe the life of a delivery driver that every night in full speed passed a bridge that was surrounded of fog - it was impossible to see what was on the bridge. The book ends with the line - this night had a truck get engine stop in the middle of the bridge
Sincerely Lasse
Maybe it become a little swenglish here (I´m a swede) I mean the fine material that will come when you stir the sand - some may call it detritus in english. But it should be from the upper 2-3 mm of the sand - not deeperThank you! What is "mulm?"
Its a risk with that because you can cause oxygen depletion in the water if you do that. The nitrification organism needs a lot of oxygen - so I would keep the skimmer on.I actually have to do a wc in the 60 so I'm glad you mentioned that I would have just tossed the water. Thanks! Lowering the ph I could just turn the skimmer off?
Nearly all things you do in this situation have backsides
Sincerely Lasse
Last edited:
Ohh I see ok thank you!Maybe it become al little swenglish here (I´m a swede) I mean the fine material that will come when you stir the sand - some may call it detritus in english. But it should be from the upper 2-3 mm of the sand - not deeper
Its a risk with that because you can cause oxygen depletion in the water if you do that. The nitrification organism needs a lot of oxygen - so I would keep the skimmer on.
Nearly all things you do in this situation have backsides
Sincerely Lasse
Similar threads
- Replies
- 9
- Views
- 243
- Replies
- 5
- Views
- 166
- Replies
- 11
- Views
- 249



















