Very good write up and interesting question.
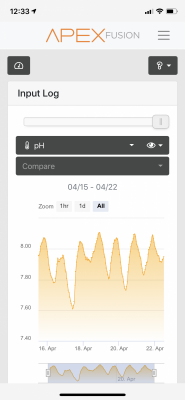
Not sure what it means but, part of my job is to record seawater parameters from a pipe line. So 60 feet down and 1000 feet out. Our PH swings everyday and also swings differently based on water temp, weather, etc.. I have never seen our ALK change. pH could be related to oxygen saturation however as that swings wildly with temperature.
Not sure what it means but, part of my job is to record seawater parameters from a pipe line. So 60 feet down and 1000 feet out. Our PH swings everyday and also swings differently based on water temp, weather, etc.. I have never seen our ALK change. pH could be related to oxygen saturation however as that swings wildly with temperature.