Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemiclean Nuked tank!
- Thread starter Knapp870
- Start date
- Tagged users None
Brew12
Electrical Gru
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Moderator Emeritus
North Alabama Reef Club
Article Administrator
My Tank Thread
I'm with you. I hope the poll question I put up gets some votes. Really curious to see the results.That's correct. Honestly my overflow piping imo adds more than enough air from the bubbles I see in sump but idk.
Yes I still struggle to see how lack of oxygen wiped out sps but all fish remained fine
Yup the fish not getting choked baffled me.That's correct. Honestly my overflow piping imo adds more than enough air from the bubbles I see in sump but idk.
Yes I still struggle to see how lack of oxygen wiped out sps but all fish remained fine
- Joined
- Jul 20, 2016
- Messages
- 329
- Reaction score
- 173
Why would fish show no affects but sps and some euphyllia die?You would want to use a pump that is rated for your size tank. If you have an 80g, I'd want a pump for 80g.
Was there an outside source? Maybe you're looking too deeply into the ChemiClean and not into other contributing factors? Did you clean the house that day? Did you touch something prior to playing in the tank? Do you have some sort of corroding metal you can see? Idk if this helps just trying to maybe solve your mystery
- Joined
- Jul 20, 2016
- Messages
- 329
- Reaction score
- 173
Thanks I appreciate the outside the box thinking but honestly I think the sensitivity of people cleaning and reaching in tanks with possible contaminants is fairly overrated at least in my experience but maybe I've been lucky in that aspect. I've never really been super careful and never had any issues. No, nothing corrosive no cleaning that I remember.Was there an outside source? Maybe you're looking too deeply into the ChemiClean and not into other contributing factors? Did you clean the house that day? Did you touch something prior to playing in the tank? Do you have some sort of corroding metal you can see? Idk if this helps just trying to maybe solve your mystery
Sorry to hear that the polyps are sloughing off.
There is a video of Julian Sprung talking about Palyotoxin at RAP this year. In it he describes a poisioning event where he nuked three tanks and himself with some palys, just by sticking his hands into the colony for a moment and disturbing it. His description of the SPS that were nuked sounded pretty much what your's look like in your pictures. Complete and total loss of tissue. Some species of zoas contain palyotoxin, some don't. You have a LOT of them in your photos. The chemiclean very well may have made them think they needed to release a toxin.
Tough call to make an absolute diagnosis in such complicated ecosystems without being intimately involved in the history and operation of that particular ecosystem.
There is a video of Julian Sprung talking about Palyotoxin at RAP this year. In it he describes a poisioning event where he nuked three tanks and himself with some palys, just by sticking his hands into the colony for a moment and disturbing it. His description of the SPS that were nuked sounded pretty much what your's look like in your pictures. Complete and total loss of tissue. Some species of zoas contain palyotoxin, some don't. You have a LOT of them in your photos. The chemiclean very well may have made them think they needed to release a toxin.
Tough call to make an absolute diagnosis in such complicated ecosystems without being intimately involved in the history and operation of that particular ecosystem.
- Joined
- Jun 9, 2016
- Messages
- 2,126
- Reaction score
- 2,076
Thanks I appreciate the outside the box thinking but honestly I think the sensitivity of people cleaning and reaching in tanks with possible contaminants is fairly overrated at least in my experience but maybe I've been lucky in that aspect. I've never really been super careful and never had any issues. No, nothing corrosive no cleaning that I remember.
Checking this thread throughout the day to see how the situation had progressed has brought me to the conclusion that we are a great bunch of people. Our dedication to this hobby is more apparent than ever when one of us has a loss like this.
I'm not entirely sure there's any blame to be had here, either on Knapp's part or the company's product. What I am certain of is we all love these wee beasties we keep. Their continued health and wellbeing is our sole purpose as a collective, and sharing your experience with us has given our group greater understanding and some pretty important warnings.
To my friend I say this, drink a beer if you choose, and forgive yourself. Loss in of itself will make you a better reefer through the experience. You can't bring them back once they're gone, but like everything on our planet, the bones can be a new home to others.
I did not say that in anything that I have receivedI still think it's erythromycin cetyl sulfate.
Last edited:
You didn't have to. 
I did not say that in anything that I have received and I work for FDA
But still being said if I did you this product I would use it at lower doses with boiling water cool to a mild temperature and then those with low doses for longer periods of timeI did not say that in anything that I have received and I work for FDA
That was simply kind and amazingChecking this thread throughout the day to see how the situation had progressed has brought me to the conclusion that we are a great bunch of people. Our dedication to this hobby is more apparent than ever when one of us has a loss like this.
I'm not entirely sure there's any blame to be had here, either on Knapp's part or the company's product. What I am certain of is we all love these wee beasties we keep. Their continued health and wellbeing is our sole purpose as a collective, and sharing your experience with us has given our group greater understanding and some pretty important warnings.
To my friend I say this, drink a beer if you choose, and forgive yourself. Loss in of itself will make you a better reefer through the experience. You can't bring them back once they're gone, but like everything on our planet, the bones can be a new home to others.
I used it for a break out on my 125 gal and in 12 hours killed every coral and 15 of my 18 fish. Never again
I used it in a 10 gallon tank that I had got some zoa's from aquarium depot had the frags for 2 months they never opened started getting over run by cyno so I treated the tank as per the directions but never did the water changes or retreat. cyno disappeared zoa's all opened up and 4 months later still doing fine.In 48 hours chemiclean has almost ruined my entire tank. Sps are whiting out zoas may be ok but closed. Used 8 spoon fulls for a 75 gallon tank with 30 gallon sump. All I was trying to do was clean things a little, no major outbreak of anything! I'm absolutely sick


How exactly does the chemiclean work. clearly there is some misunderstanding.
I did not have time to read all 13 pages so if this ended up being covered please forgive the duplicate reply. Chemiclean is not an antibiotic at all, at least in the traditional sense like erythromycin or penicillin. I believe I read some where it is mostly likely potassium permanganate or something similar, which is a strong oxidizing agent, similar to hydrogen peroxide but has a much higher affinity to grab electrons from things. Most animals have good defenses against oxygen radicals and other oxidizing molecules (why hydorgen peroxide bubble when we put it on a wound). Many plants and photosynthetic organisms rely on light to break water apart and moving electrons down a chain of molecules to form sugar and oxygen. If one adds a strong oxidizing agent you will get a result that is similar to something many of us are familiar with too when we give our corals to much light (=bleaching corals) the chlorophyll and auxiliary carotenoid pigments (which protect the chlorophyll from to much light energy) will begin to form triplet-triplet electron transfers between chlorophyll and the protective carotenoid pigments which will produce singlet oxygen molecules if allowed to go on for to long this is also know as a free radical. This singlet oxygen is a powerful oxidizer and will destroy the chlorophyll and kill the organism as a result. By adding the chemiclean we are simply adding a chemical that will react with electron rich area like the photocenters of bacteria that are very susceptible to this type of chemical. This is similar to the work @Twillard is doing with hydrogen peroxide, which is just a weaker oxidizing agent and he has shown will kill some cyanobacteria that are more susceptible and can be killed with H2O2. My cyano was unfortunately not susceptible to H2O2 and I ended up going the chemiclean route.
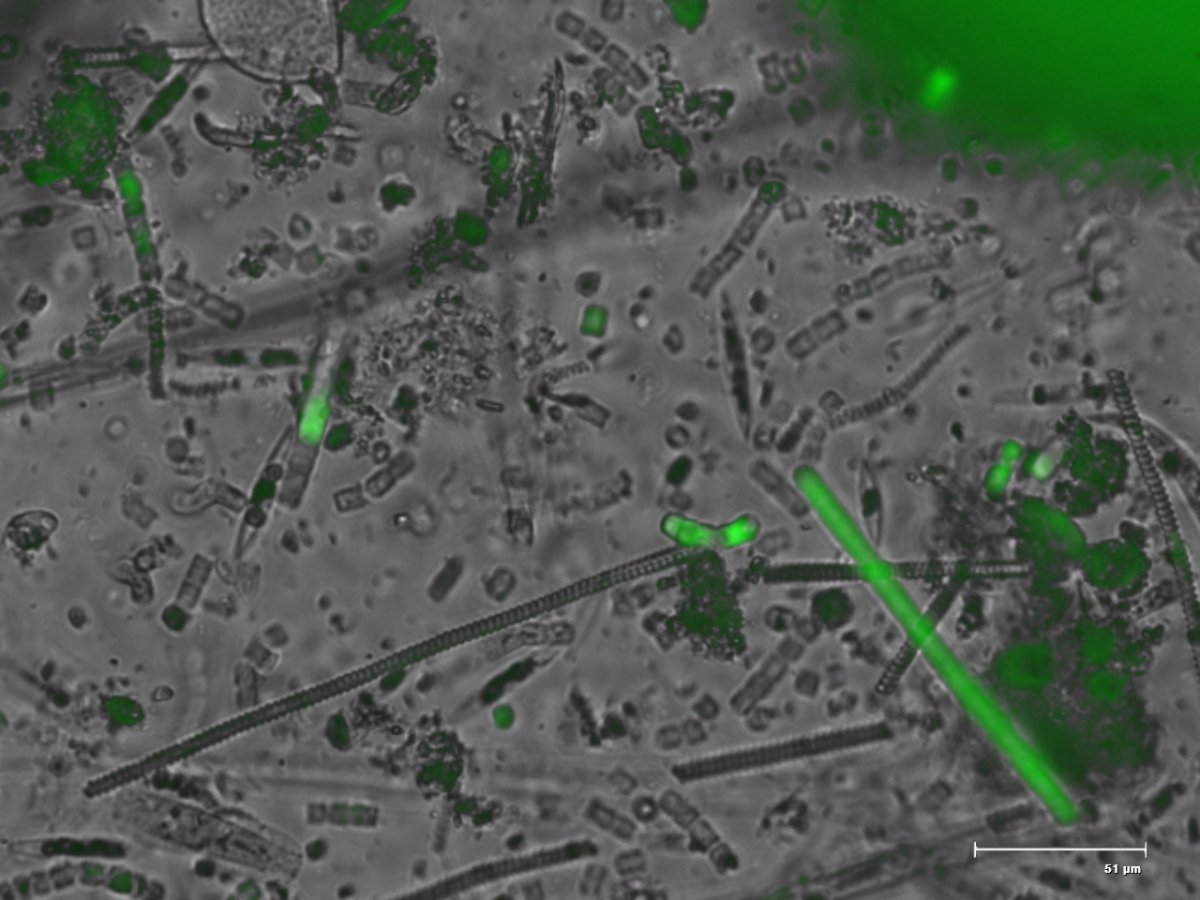
I used chemiclean and oxygenated like mad a few hours before dosing and continued until I was pretty certain I had removed it with carbon, waterchange, and lots of wet skimate production. I took what remained of the cyano matt from my sand bed to work and put it under one of our fluorescent microscopes. When excited with certain wavelengths of blue light carentoids and chlorophyll will fluoresce green. I attached the image which was approximately 72 post treatment. What you can see is most of the cells no longer are fluorescing green, there are a few tough ones that held on for a couple of days longer, it has been about 4 weeks post treatment and it has not come back so the holdovers most likely perished a few days later. Really what this indicates is it attacks the photocenters of the bacteria and as a result kills them, also why they can claim is has little effect on the beneficial bacteria.
Corals and animals have mechanisms to protect against oxidizing agents better than plants. This is why H202 at 3% applied to algae on a rock will kill it, notice it bleaches out then sort of disintegrates away. This is how I am winning finally against bryopsis. We just can't add H2O2 to our tanks at a final concentration of 3% and not begin to harm our fish, this would probably begin to burn their gills at this strength. Now if your light levels are near the max the corals can tollerate to the point they are near the edge, called photosaturation where the chlorophyll is right at the edge of producing singlet oxygen, when this balance is tipped over it's called photoinhibition where the singlet oxygen begins taking out chlorophyll molecules. When this happens in a corals tissue the coral attempts to aid the zooxanthellae by producing anti-oxidants, if this does not help enough the coral is left with no other option except to eject the zooxanthellae and the result is bleaching of the coral. This might be what is happening in the OP tank where the chemiclean may have pushed the equation for some his corals over this boundary.
Might be a good idea to reduce the light intensity for a week or so as often if the coral is not dead, just bleached they can recolonize with zooxanthellae once conditions are right again. Anyway hope this sheds some light on how this stuff works. I had not bad effects form using it, but I also know how much light at peak lighting my corals get and am confident they are well below the photosaturation point. The last part is theory as to what occurred in the OP's tank and might help by reducing the lighting for a few days. Also run as much carbon as you can in the reactor. I used about 4 cups for approx 150 gallons of known water volume, and probably skimmed off 2 more gallons along with a 25% water change.
Hope things recover for you!
I love you.I did not have time to read all 13 pages so if this ended up being covered please forgive the duplicate reply. Chemiclean is not an antibiotic at all, at least in the traditional sense like erythromycin or penicillin. I believe I read some where it is mostly likely potassium permanganate or something similar, which is a strong oxidizing agent, similar to hydrogen peroxide but has a much higher affinity to grab electrons from things. Most animals have good defenses against oxygen radicals and other oxidizing molecules (why hydorgen peroxide bubble when we put it on a wound). Many plants and photosynthetic organisms rely on light to break water apart and moving electrons down a chain of molecules to form sugar and oxygen. If one adds a strong oxidizing agent you will get a result that is similar to something many of us are familiar with too when we give our corals to much light (=bleaching corals) the chlorophyll and auxiliary carotenoid pigments (which protect the chlorophyll from to much light energy) will begin to form triplet-triplet electron transfers between chlorophyll and the protective carotenoid pigments which will produce singlet oxygen molecules if allowed to go on for to long this is also know as a free radical. This singlet oxygen is a powerful oxidizer and will destroy the chlorophyll and kill the organism as a result. By adding the chemiclean we are simply adding a chemical that will react with electron rich area like the photocenters of bacteria that are very susceptible to this type of chemical. This is similar to the work @Twillard is doing with hydrogen peroxide, which is just a weaker oxidizing agent and he has shown will kill some cyanobacteria that are more susceptible and can be killed with H2O2. My cyano was unfortunately not susceptible to H2O2 and I ended up going the chemiclean route.
I used chemiclean and oxygenated like mad a few hours before dosing and continued until I was pretty certain I had removed it with carbon, waterchange, and lots of wet skimate production. I took what remained of the cyano matt from my sand bed to work and put it under one of our fluorescent microscopes. When excited with certain wavelengths of blue light carentoids and chlorophyll will fluoresce green. I attached the image which was approximately 72 post treatment. What you can see is most of the cells no longer are fluorescing green, there are a few tough ones that held on for a couple of days longer, it has been about 4 weeks post treatment and it has not come back so the holdovers most likely perished a few days later. Really what this indicates is it attacks the photocenters of the bacteria and as a result kills them, also why they can claim is has little effect on the beneficial bacteria.
Corals and animals have mechanisms to protect against oxidizing agents better than plants. This is why H202 at 3% applied to algae on a rock will kill it, notice it bleaches out then sort of disintegrates away. This is how I am winning finally against bryopsis. We just can't add H2O2 to our tanks at a final concentration of 3% and not begin to harm our fish, this would probably begin to burn their gills at this strength. Now if your light levels are near the max the corals can tollerate to the point they are near the edge, called photosaturation where the chlorophyll is right at the edge of producing singlet oxygen, when this balance is tipped over it's called photoinhibition where the singlet oxygen begins taking out chlorophyll molecules. When this happens in a corals tissue the coral attempts to aid the zooxanthellae by producing anti-oxidants, if this does not help enough the coral is left with no other option except to eject the zooxanthellae and the result is bleaching of the coral. This might be what is happening in the OP tank where the chemiclean may have pushed the equation for some his corals over this boundary.
Might be a good idea to reduce the light intensity for a week or so as often if the coral is not dead, just bleached they can recolonize with zooxanthellae once conditions are right again. Anyway hope this sheds some light on how this stuff works. I had not bad effects form using it, but I also know how much light at peak lighting my corals get and am confident they are well below the photosaturation point. The last part is theory as to what occurred in the OP's tank and might help by reducing the lighting for a few days. Also run as much carbon as you can in the reactor. I used about 4 cups for approx 150 gallons of known water volume, and probably skimmed off 2 more gallons along with a 25% water change.
Hope things recover for you!
- Joined
- Jul 20, 2016
- Messages
- 329
- Reaction score
- 173
Thank you! This is very possible as I am running led. I wish I would haveeft them off or reduced... Maybe too late now...I did not have time to read all 13 pages so if this ended up being covered please forgive the duplicate reply. Chemiclean is not an antibiotic at all, at least in the traditional sense like erythromycin or penicillin. I believe I read some where it is mostly likely potassium permanganate or something similar, which is a strong oxidizing agent, similar to hydrogen peroxide but has a much higher affinity to grab electrons from things. Most animals have good defenses against oxygen radicals and other oxidizing molecules (why hydorgen peroxide bubble when we put it on a wound). Many plants and photosynthetic organisms rely on light to break water apart and moving electrons down a chain of molecules to form sugar and oxygen. If one adds a strong oxidizing agent you will get a result that is similar to something many of us are familiar with too when we give our corals to much light (=bleaching corals) the chlorophyll and auxiliary carotenoid pigments (which protect the chlorophyll from to much light energy) will begin to form triplet-triplet electron transfers between chlorophyll and the protective carotenoid pigments which will produce singlet oxygen molecules if allowed to go on for to long this is also know as a free radical. This singlet oxygen is a powerful oxidizer and will destroy the chlorophyll and kill the organism as a result. By adding the chemiclean we are simply adding a chemical that will react with electron rich area like the photocenters of bacteria that are very susceptible to this type of chemical. This is similar to the work @Twillard is doing with hydrogen peroxide, which is just a weaker oxidizing agent and he has shown will kill some cyanobacteria that are more susceptible and can be killed with H2O2. My cyano was unfortunately not susceptible to H2O2 and I ended up going the chemiclean route.
I used chemiclean and oxygenated like mad a few hours before dosing and continued until I was pretty certain I had removed it with carbon, waterchange, and lots of wet skimate production. I took what remained of the cyano matt from my sand bed to work and put it under one of our fluorescent microscopes. When excited with certain wavelengths of blue light carentoids and chlorophyll will fluoresce green. I attached the image which was approximately 72 post treatment. What you can see is most of the cells no longer are fluorescing green, there are a few tough ones that held on for a couple of days longer, it has been about 4 weeks post treatment and it has not come back so the holdovers most likely perished a few days later. Really what this indicates is it attacks the photocenters of the bacteria and as a result kills them, also why they can claim is has little effect on the beneficial bacteria.
Corals and animals have mechanisms to protect against oxidizing agents better than plants. This is why H202 at 3% applied to algae on a rock will kill it, notice it bleaches out then sort of disintegrates away. This is how I am winning finally against bryopsis. We just can't add H2O2 to our tanks at a final concentration of 3% and not begin to harm our fish, this would probably begin to burn their gills at this strength. Now if your light levels are near the max the corals can tollerate to the point they are near the edge, called photosaturation where the chlorophyll is right at the edge of producing singlet oxygen, when this balance is tipped over it's called photoinhibition where the singlet oxygen begins taking out chlorophyll molecules. When this happens in a corals tissue the coral attempts to aid the zooxanthellae by producing anti-oxidants, if this does not help enough the coral is left with no other option except to eject the zooxanthellae and the result is bleaching of the coral. This might be what is happening in the OP tank where the chemiclean may have pushed the equation for some his corals over this boundary.
Might be a good idea to reduce the light intensity for a week or so as often if the coral is not dead, just bleached they can recolonize with zooxanthellae once conditions are right again. Anyway hope this sheds some light on how this stuff works. I had not bad effects form using it, but I also know how much light at peak lighting my corals get and am confident they are well below the photosaturation point. The last part is theory as to what occurred in the OP's tank and might help by reducing the lighting for a few days. Also run as much carbon as you can in the reactor. I used about 4 cups for approx 150 gallons of known water volume, and probably skimmed off 2 more gallons along with a 25% water change.
Hope things recover for you!

It is due to O2. I have never run chemiclean at full strength. I always have started out at half the recommended amount if I ever saw the start of cyano and never got a full outbreak. Typically controlled nutrients and only had to dose one at start of outbreak.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,498
- Reaction score
- 63,897
Co2 lowers pH and dissolved o2 increases. PH never dropped below 8.1
I haven't read the whole thread, but i want to clarify that.
Change is CO2 do not necessarily have any impact on O2. High or low CO2 does not imply or cause high or low O2.
Similar threads
- Replies
- 17
- Views
- 244
- Replies
- 9
- Views
- 101
- Replies
- 1
- Views
- 63
- Replies
- 62
- Views
- 678

















